Introduction: Right ventricle (RV) size and function are important for the prognosis of patients with various forms of cardiovascular disease. The right heart differs from the left in terms of anatomy and physiology. COVID-19 may present with cardiac manifestations including myocardial injury, heart failure and cardiac arrhythmias. In Chinese experience, patients with cardiovascular disease (CVD) have case fatality rate (CFR) is 10.5%. Proposed mechanisms of myocardial injury included impaired physiologic reserve (cardiovascular and pulmonary), impaired immune response, augmented inflammatory response, vulnerability to SARS-CoV-2-induced endothelial dysfunction, and effects mediated by the angiotensin-converting enzyme 2 receptor.
Material and method: We were retrospectively studied adult patients admitted between March 01, 2020, and septumber,15, 2021, in Hamad Medical Cooperation COVID-19 infection facility hospitals. Demographic data, comorbid conditions, medications, physical examination, laboratory findings, chest x ray, transthoracic echocardiography (TTE) were recorded. All patients underwent comprehensive transthoracic echocardiography during hospital stay with base on clinical cardiac symptoms or ECG changes. Evaluation of left and right ventricle (RV) systolic function used by Left ventricular ejection fraction (LVEF), tricuspid annular plane systolic excursion (TAPSE) and ratio of tricuspid annular plane systolic excursion/systolic pulmonary artery pressure (TAPSE/PASP).
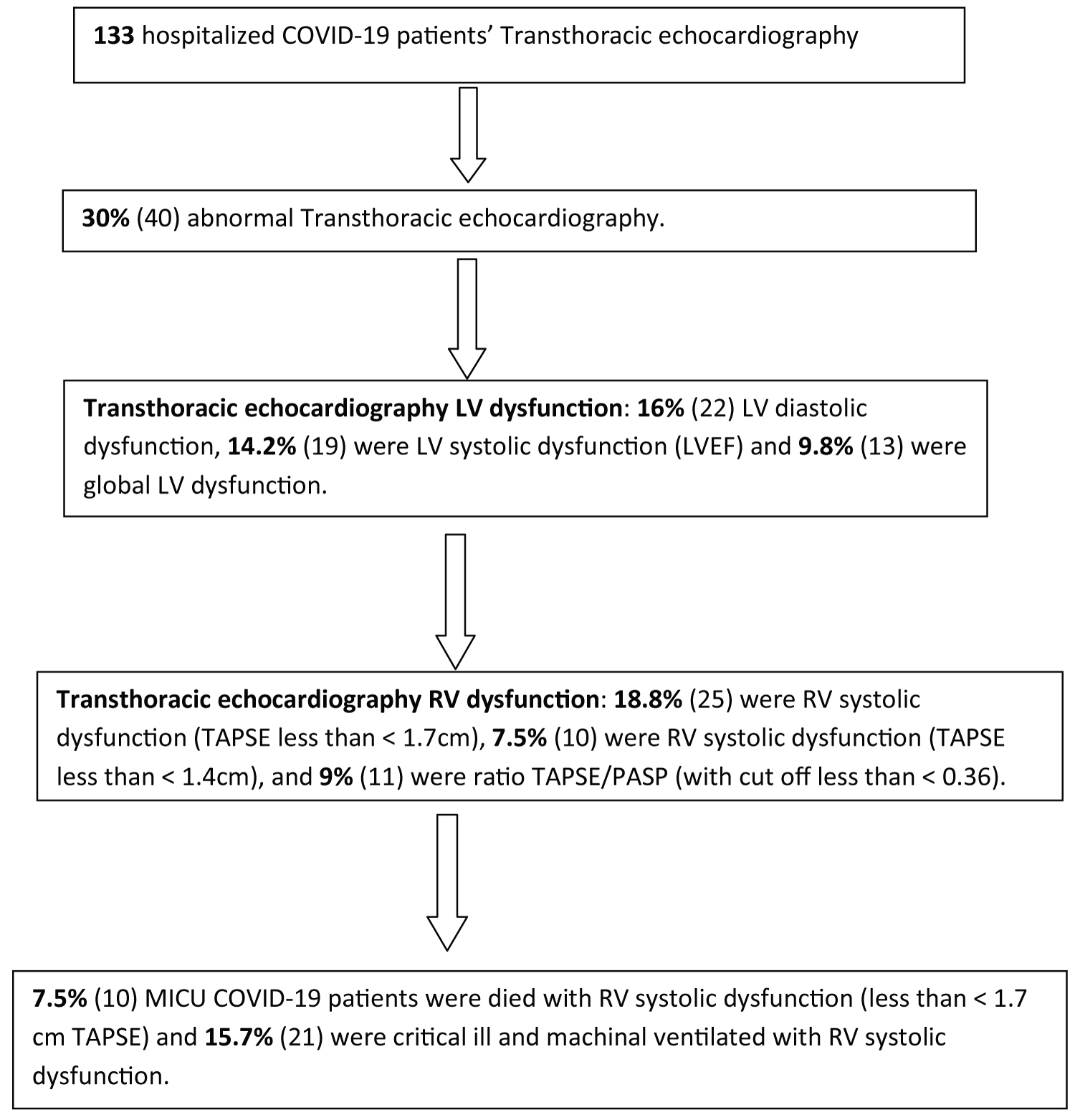
Result: 133 hospitalized patients with confirmed SARS CoV-2 infection were included in this study. The most comorbid illness was diabetes (62), hypertension, obesity with > 30 BMI (28). The majority of COVID-19 patients in this study were severe and critical ill pneumonia in this study. 53.4% (71) were acute myocardial injury and 26.3% (35) were acute heart failure. 30% (40) were abnormal transthoracic echocardiography included; 18.8% (25) were RV systolic dysfunction, 14.2% (19) were LV systolic dysfunction (LVEF), 12.8% (17) were preserved LVEF with RV systolic dysfunction. 15.7% (21) were critical ill and machinal ventilated with RV systolic dysfunction and 7.5% (10) patients died with RV systolic dysfunction (less than < 1.7 cm TAPSE). 93.5% (124) patients admitted in MICU, 28% (37) patients were died in MICU with covid complication and 7.5% (10) patients died with RV dysfunctions.
Discussion: Right ventricle (RV) size and function are important for the prognosis of COVID-19 related various forms of cardiovascular disease. Cardiac complications of COVID-19 included myocardial injury, heart failure (HF), cardiogenic shock, multisystem inflammatory syndrome in adult (Kawasaki-like illness) and cardiac arrhythmias included sudden cardiac arrest. An international survey of Echo, majority finding of COVID-19 patients included 39% were LV abnormalities, 33% were RV abnormalities, 15% were right ventricular dilatation, 8% were elevated pulmonary artery pressures. In this study, one third COVID-19 hospitalized patients transthoracic echo (TTE) were abnormal included; 18.8% were RV systolic dysfunction, 14.2% were LV systolic dysfunction, 9.8% were global LV dysfunction and 12.8% were RV systolic dysfunction with preserved LV systolic function (LVEF). In comparison with LV systolic dysfunction, RV systolic dysfunction was significantly higher (18.8% Vs 14.2%).
Conclusions: The most important consequence of severe COVID-19 pneumonia is RV failure in contrast to LV failure with elevated troponin and brain natriuretic peptide (BNP). RV failure is independent predictor of severity and mortality of COVID-19 pneumonia. This study helps physicians to look RV failure in COVID-19 pneumonia as this is a marker of higher morbidity and mortality.
Right ventricle (RV), Case fatality rate (CFR), Heart failure (HF), Transthoracic echocardiography (TTE).
Right ventricle (RV) size and function are important for the prognosis of patients with various forms of cardiovascular disease. RV dysfunction is associated with excess morbidity and mortality in patients with chronic left-sided heart failure, acute myocardial infarction (with or without RV infarction), pulmonary embolism, pulmonary arterial hypertension, and congenital heart disease [1]. The right heart differs from the left in terms of anatomy and physiology. The RV loosely resembles a pyramid and is composed of three portions named the inlet, the body, and the outflow tract. Contraction is generated by a deep layer of longitudinal fibers that result in longitudinal (base to apex) shortening, and a superficial layer of circumferential fibers that result in inward thickening [2]. The RV lacks a third layer of spiral fibers as seen in the left ventricle. The RV end-diastolic volume is slightly larger than that of the left ventricle, and as a result has a slightly lower ejection fraction to generate the same stroke volume.
COVID-19 typically present with respiratory tract infection, but may present with cardiac manifestations including myocardial injury, heart failure and cardiac arrhythmias. In fact, mortality is greater in covid-19 patients with myocardial injury than those without (59.6% vs. 8.9%) [3]. Recent studies have shown that the incidence of acute myocardial injury in hospitalized patients with critical ill COVID-19 is 20% [4]. In Chinese experience, patients with no comorbidities have a case fatality rate (CFR) of 0.9% while those with CVD have CFR of 10.5% [5]. A retrospective study demonstrated that right ventricular (RV) dilation was observed in 31% of the hospitalized patients with COVID‐19, which is significantly associated with the mortality [6].
Patients with COVID-19 may have cardiac manifestations but the causes of these findings have not been established. Possible causes of myocardial injury in patients with COVID-19 include myocarditis, hypoxic injury, stress (takotsubo) cardiomyopathy, ischemic injury caused by cardiac microvascular damage or epicardial coronary artery disease, right heart strain or acute cor-pulmonale and systemic inflammatory response syndrome (cytokine storm) [7-9]. Proposed mechanisms include impaired physiologic reserve (cardiovascular and pulmonary), impaired immune response, augmented inflammatory response, vulnerability to SARS-CoV-2-induced endothelial dysfunction, and effects mediated by the angiotensin-converting enzyme 2 receptor [10]. The aim of this retrospective observational study determines right ventricular dysfunction in COVID-19 hospitalized patients and its outcome.
We were retrospectively studied adult patients (≥ 18 years of age) admitted between March 01, 2020, and septumber,15, 2021, in Hamad Medical Cooperation COVID19 infection facility hospitals. A COVID-19 confirmed case were defined as a positive or reactive result on real-time reverse transcription polymerase chain reaction (RT-PCR) analysis of nasopharyngeal and oropharyngeal specimens from patients were tested simultaneously. This study was approved and written informed consent was waived by the Ethics Committee of HMC (MRC 01-21-848), Doha, Qatar, on 30, September, 2021, owing to retrospective nature of study and rapid emergence of the disease and the urgent need to collect data. Demographic data, comorbid conditions, medications, physical examination, laboratory findings (including CBC, CMP, PT/APPT/INR, LFT, CRP, Ferritin, LDH, D-dimer, IL-6, lactic acid, CPK, myoglobin, troponin-T levels, BNP), chest x ray, transthoracic echocardiography (TTE) were recorded. All patient admitted with or with cardiac signs and symptoms, with and without abnormal ECG changes, high troponin level suggestive of myocardial injury and abnormal Echo finding specifical for right ventricular dysfunction were included while patients known to previous chronic left-sided heart failure, old myocardial infarction (with or without RV infarction), pulmonary arterial hypertension, and congenital heart disease excluded. We defined the degree of severity of COVID-19 (severe vs mild) at the time of admission using the American Thoracic Society guidelines for community-acquired pneumonia. All patients underwent comprehensive transthoracic echocardiography during hospital stay with base on clinical cardiac symptoms or ECG changes. Echocardiography was performed in a standard manner by cardiologists with expertise in echocardiographic recording and interpretation. Evaluation of left and right ventricle (RV) systolic function used by Left ventricular ejection fraction (LVEF), tricuspid annular plane systolic excursion (TAPSE) and ratio of tricuspid annular plane systolic excursion/systolic pulmonary artery pressure (TAPSE/PASP). Severity of left systolic function graded by Left ventricular ejection fraction (LVEF) as mild (51-41%), moderate (40-30%), severe (< 30%). Right ventricle systolic function considers abnormal (RV systolic dysfunction) as TAPSE < 1.7 cm and low cut off of < 0.36 ratio of tricuspid annular plane systolic excursion/systolic pulmonary artery pressure (TAPSE/PASP). Acute myocardial injury was defined as high troponin T hs for male > 30 ng/l and for female > 20ng/l, and acute heart failure defined as NT pro BNP > 1800 pg/ml. Descriptive statistics used to summarize and determine the sample characteristics and distribution of various considered parameters related to demographic, diagnostic, clinical features, follow-up outcome measures and other related features of the patients of such patients. The normally distributed data and results reported with mean and standard deviation (SD) with corresponding 95% confidence interval (CI); the remaining results reported with median and interquartile range (IQR). Categorical data summarized using frequencies and percentages. Associations between two or more qualitative variables examined and assessed using Pearson Chi-square and Fisher Exact tests as appropriate. Unpaired t and ANOVA used to compare mean values of different quantitative parameters between two or more than two groups. Correlation between various outcomes measured evaluated quantitatively calculated by Pearson or Spearman rank-order correlation. An appropriate regression analysis performed to assess the effects of various factors and covariates on primary outcome measures. Pictorial presentations of the key results made using appropriate statistical graphs. A two-sided P value < 0.05 statistically significant. All statistical analyses done using statistical packages SPSS 24.0 (SPSS Inc. Chicago, IL) and Epi Info 2000 (Centers for Disease Control and Prevention, Atlanta, GA).
A total of 133 hospitalized patients with confirmed SARS CoV-2 infection were included in this study. Their demographic and clinical characteristics were shown in Table 1 and Table 2. Their Age mean (SD-14) was 56 (range from 28 to 84 year) years, and 92% were male (122) and 8% were female (11). 49% (65) were less than 55 years age, 51% (67) were greater than 55 years age. Different nationality with majority were 25.5% Indian (34), 13.5% were Filipino (18), 11.2% were Bangladeshi (15) and Pakistani (15), 7.5% were Qatari (10) and Nepali (10). The most common symptoms at onset of illness included fever were 80.5% (107), cough was 73% (97), short of breathing were 70.7% (94), myalgia was 22% (29), headache was 5.3% (7), chest pain was 4.5% (6). Of all patients, following underlying risk factors included 46.6% were diabetes (62), 46.2% were hypertension (61), 21% obesity with > 30 BMI (28), 12.9% newly diagnosed (17), 8.3% were pre-diabetic (11), 6.1% were chronic kidney disease (8), 6.1% were ischemic heart disease (8), 4.5% were asthma (6) and 1.6% were smoker (2) as shown in Table 3. According to the American Thoracic Society guidelines for community-acquired pneumonia, 12% patients (16) were severe infection, 81% (107) were critically ill and 7% (10) were mild pneumonia. CT pulmonary angiography showed of 31.6% (42) patients were positive pulmonary embolism with majority at segmental (24.8%), subsegmental (5.7%) and pulmonary artery level (5.7%). 6.7% (9) patient were both pulmonary embolism and right ventricle dysfunction and 5.2% (7) patient were bilateral RV and LV dysfunction. 79% (105) patients admitted in MICU were intubated and ventilated while 14.5% (19) patients used high flow nasal cannula. 28% (37) patients were died in MICU with covid complication and 7.5% (10) patients died with RV dysfunctions.
Table 1: Demographics. View Table 1
Table 2: Clinical manifestations. View Table 2
Table 3: Underlying risk factors. View Table 3
Other multiorgan systems involvement of covid-19 patients including 74.4% (99) were skeletal muscle injury (serum creatine kinase > 300U/L), 53.4% (71) acute myocardial injury (high tropT hs, male > 30, female > 20 ng/l), 50.4% (67) had acute liver injury (> 200 ALT or AST levels), 44.4% (59) were acute kidney injury (creatinine > 106 umol/l and urea > 8 mmol/l), 30% (40) patient were greater than > 6.5 HbA1c, 26.3% (35) were acute heart failure (high NT-proBNP > 1800 pg/mL), 8.3% (11) were acute pancreatitis (serum lipase >180 u/l), 3.8% (5) had previous uncontrolled blood sugar (with HbA1c > 12) as shown in Table 4. Laboratory features associated with severe COVID-19 including 95.5% (127) had > 500 mcg/L ferritin level, 96.2% (128) had >245 units/L LDH, 91.7% (122) had > 100 mg/l C-reactive protein levels, 94% (125) had > 3000 ng/mL D-dimer, 68.4% (91) had > 70 pg/m of IL-6, 74.4% (99) had > 300u/L serum creatine kinase, 51.1% (68) had > 2ng/ml procalcitonin, 9.8% (13) had > 4 mmol/l lactic acid as shown in Table 4.
Table 4: Laboratory features for severe COVID-19. View Table 4
Transthoracic echocardiography was performed 133 patients as shown in Table 5, 70% (93) were normal, 30% (40) were abnormal findings included; 14.2% (19) were LV systolic dysfunction (LVEF), with mild EF were 6% (8), moderate EF were 7.5% (10) and severe were 0.9% (1), 16% (22) LV diastolic dysfunction and 9.8% (13) were global LV dysfunction. RV systolic dysfunction measured by TAPSE included less than < 1.7 cm were 18.8% (25) and < 1.4 cm were 7.5% (10), and ratio of tricuspid annular plane systolic excursion/systolic pulmonary artery pressure (TAPSE/PASP) with cut off less than < 0.36 were 9% (11). The high right atrial pressure was 65.4% (87) and high right ventricular systolic pressure (RVSP) value were 18.9% (25). 12.8% (17) were preserved LVEF with RV systolic dysfunction and 5.2% (7) were impaired LVEF with RV systolic dysfunction as shown in Table 5. There were 15.7% (21) were critical ill and machinal ventilated with RV systolic dysfunction and 7.5% (10) patients died with RV systolic dysfunction (less than < 1.7 cm TAPSE).
Table 5: Echocardiographic findings. View Table 5
This is first research article on right ventricular dysfunction in COVID-19 patients and its impact on mortality of hospitalized patients from Qatar. Right ventricle (RV) size and function are important for the prognosis of COVID-19 related various forms of cardiovascular disease. COVID-19 present with a broad spectrum of asymptomatic or symptomatic cardiac manifestations. Cardiac complications include myocardial injury, heart failure (HF), cardiogenic shock, multisystem inflammatory syndrome in adult (Kawasaki-like illness) and cardiac arrhythmias included sudden cardiac arrest [11]. Myocardial injury is common among COVID-19 patients and defined as elevated cardiac troponin levels. Clinical conditions associated with myocardial injury include myocarditis, stress cardiomyopathy, and myocardial infarction [12,13]. Heart failure in patients with COVID-19 may be precipitated by acute illness in patients with preexisting heart disease, acute hemodynamic stress, or incident acute myocardial injury [14]. A study of hospitalized COVID-19 patients at a hospital in New York found that a history of heart failure (HF) was associated with adverse outcomes, including longer length of stay (8 versus 6 days), increased risk of mechanical ventilation (22.8 versus 11.9%; adjusted OR 3.64, 95% CI 2.56-5.16), and mortality (40.0 versus 24.9%; adjusted OR 1.88, 95% CI 1.27-2.78) [15]. Viral myocarditis caused by SARS-CoV-2 has not been definitively confirmed by myocardial histologic and viral genome analysis [16]. Transthoracic echocardiography (TTE) findings in patients with COVID-19 by an international survey included data on 1216 patients from 69 countries; 55% of patients had an abnormal TTE, included LV abnormalities in 39% with echo evidence of new acute myocardial infraction (3%), myocarditis (3%), stress cardiomyopathy (3%) and RV abnormalities were 33%, right ventricular dilatation were 15%, elevated pulmonary artery pressures were 8% and 1% were cardiac tamponade and endocarditis [17]. In this retrospective observational study, majority of patients were admitted in medical intensive care unit (ICU) with severe and critically ill covid pneumonia (93%). One third COVID-19 hospitalized patients transthoracic echo (TTE) were abnormal (30%) included; 18.8% were RV systolic dysfunction, 14.2% were LV systolic dysfunction, 9.8% were global LV dysfunction and 12.8% were RV systolic dysfunction with preserved LV systolic function (LVEF). In comparison with LV systolic dysfunction, RV systolic dysfunction was significantly higher (18.8% vs. 14.2%). There was significant higher (double) RV systolic dysfunction in patients with preserved LV systolic function (LVEF) as comparison with impaired LV systolic dysfunction (12.8% vs. 5.2%). There was other multiorgan system involvement of COVID-19 infection included substantial higher acute myocardial injury with elevated troponin T level (53.4%). The overall mortality due to COVID-19 in this study were 28%, while mortality in patient with RV systolic dysfunction were less 7.5% (28% vs. 7.5%). In comparison with available lectures review with this study, there were considerably less frequent RV systolic dysfunction (18.8% vs. 33%) and mortality (7.5% vs. 40%). The results of this study suggest that physician should consider to look signs of RV failure in echocardiography as this is independent predictor of severe COVID-19 infection and higher mortality.
The most important consequence of severe COVID-19 pneumonia on cardiac structure and function is RV failure in contrast to LV failure with elevated troponin and brain natriuretic peptide (BNP). RV failure is independent predictor of severity and mortality of COVID-19 pneumonia. This study helps physicians to look RV failure in COVID-19 pneumonia as this is a marker of higher morbidity and mortality.
Entitled "Right Ventricular Dysfunction in COVID-19 Patients and its Impact on Mortality" has been approved by MRC.
There is no conflict of interest.
Nil.
Liaquat Ali: Data collection, data analysis, manuscript writing & review literature.
Mohammad Sharif: Manuscript writing, manuscript review.
Mohammad Naeem: Manuscript writing & review literature.
Mohammad Imran: Data analysis, manuscript review.
Imran Janjua: Data analysis, manuscript writing & review.
Kazi Muntaha: Data analysis, manuscript writing & review.
Ameena Chalil: Data collection, data analysis.
Mirza Baig: Manuscript writing, manuscript review.
Hanna Ali: Data analysis, manuscript writing.
Hana Aweida: Data analysis, manuscript writing.
Ambreen Iqrar: Manuscript writing, manuscript review.
Yasin Zada: Data collection, data analysis.