Polymyositis (PM) is a systemic inflammatory disease affecting muscles and several other organs, including gastrointestinal system, in which the main symptom is dysphagia.
Here, we describe a case of a 57-years-old woman presenting a difficult-to-manage dysphagia related to polymyositis, treated with benefit with Subcutaneous Immunoglobulins (SCIg). Beyond symptomatic therapies, immunosuppressive therapies (glucocorticoids, methotrexate, azathioprine, mycophenolatemofetil, and cyclosporine) are the main treatment of PM related dysphagia. Nowadays, few data are available to evaluate effectiveness and safety of intravenous immunoglobulins on esophageal involvement in refractory PM and only anecdotal cases are reported about the use of SCIg in the treatment of dysphagia in inflammatory myopathies (including inclusion body myositis). Up to date, this is the second observation describing an improvement of dysphagia in a subject affected by PM treated with SCIg; further studies are needed to evaluate the effectiveness of SCIg in the management of these patients.
Polymyositis, Dysphagia, Therapy, Subcutaneous immunoglobulins
Polymyositis (PM) is a systemic inflammatory disease affecting muscles and several other organs, including gastrointestinal system. Dysphagia is the main symptom of gastrointestinal involvement, due to weakness of oropharyngeal and esophageal muscles; it can sometimes affect seriously the quality of life and can be refractory to traditional immunosuppressive and symptomatic treatments [1].
To date, few data are available to evaluate effectiveness and safety of Intravenous Immunoglobulins (IVIg) on esophageal involvement and multiple mechanisms of action have been proposed, such as interference with complement and membrane attack complex deposition, down regulation of intercellular adhesion molecule 1 in several cell types, down regulation of TGFβ1 expression in muscle fibers [2].
Here we describe a case of a woman presenting dysphagia related to polymyositis particularly complicated to manage.
A 57-year-old woman, affected by polymyositis since 2003, was referred to our Rheumatology Unit for a severe dysphagia despite the ongoing therapy (low doses of steroids and Methotrexate [MTX] 7.5 mg weekly).
At the onset, she presented severe muscle weakness, elevation of the Creatin-Kinase (CK) level (reaching 350 IU/L), Raynaud phenomenon, calcinosis, sicca syndrome, dysphagia, and gastric pyrosis, positivity for Antinuclear Antibodies (ANA) 1:160 speckled with no anti-Extractable Nuclear Antibodies (ENA). The patient underwent to a muscle biopsy showing changes in fibre size, myofiber degeneration and regeneration with diffuse or focal inflammatory infiltrates and a electromyography suggestive of myopathy. Finally, other connective tissue diseases, such as systemic sclerosis or mixed connective tissue disease were excluded; myositis-specific antibodies including anti-SRP were negative and the patient didn't take statins. Nail fold video capillaroscopy was negative for scleroderma-like pattern [3], while a high-resolution chest tomography and the pulmonary function tests excluded an interstitial lung disease.
In 2008 she firstly referred to our Centre, because of a worsening of gastric pyrosis, dysphagia, and the appearance of dyspepsia, postprandial vomiting, constipation and progressive weight loss (5 kg lost in 10 months, reaching 45 kg), with a Number Rating Scale (NRS) of gastrointestinal symptoms of 5/10.
At the admission, she showed a mild increase of CK 257 IU/L (normal value < 145), asthenia and muscle weakness. We increased MTX to 20 mg, but the patient showed a worsening in vomiting and nausea, probably due to the MTX itself. We associated only a low dosage of metylprednisone, since the patient already presented glucose intolerance and osteoporosis.
Therefore, to obtain a better disease control and a better compliance, we started monthly IVIg, 2 g/kg in 3 days, associated to Methotrexate 15 mg weekly and metylprednisone 8 mg daily. The patient also maintained therapy with prokinetics and proton-pump inhibitors.
Her muscle strength, asthenia and dysphagia (NRS 2/10) rapidly improved and CK levels progressively decreased, reaching normal values after 12 months (130 IU/L); IVIg was progressively discontinued and, after 2 years, we were able to reduce MTX dosage to 10 mg/week and stop therapy with steroids. The clinical situation remained stable, with acceptable quality of life, for a long time (Figure 1).
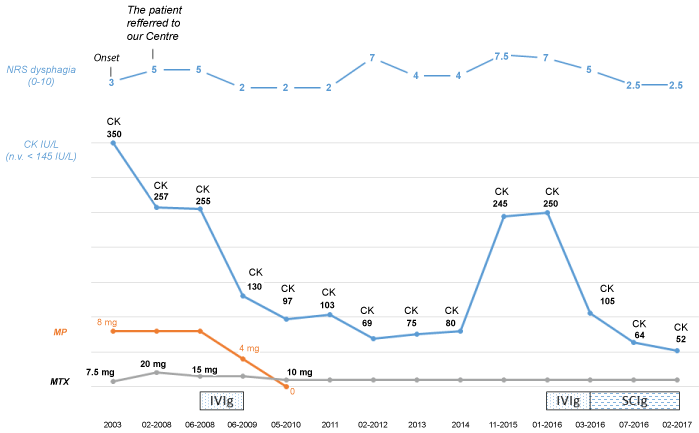 Figure 1: NRS: Number Rating; CK: Creatin-Kinase; IU/L: International Units per Liter; n.v.: normal value; MTX: Methotrexate; MP: Methylprednisone; IVIg: International Immunoglobulins; SCIg: Subcutaneous Immunoglobulins. View Figure 1
Figure 1: NRS: Number Rating; CK: Creatin-Kinase; IU/L: International Units per Liter; n.v.: normal value; MTX: Methotrexate; MP: Methylprednisone; IVIg: International Immunoglobulins; SCIg: Subcutaneous Immunoglobulins. View Figure 1
In 2012, because of a worsening of the gastroenteric involvement (NRS 7/10), she underwent to esophageal manometry, and video fluoroscopy swallow study detecting esophageal acalasia, gastroesophageal reflux, esophagitis and motor-like dyspepsia gastroparesis. Prokinetic agents, Pyridostigmine, Trimebutine, H2 receptor antagonists or Proton-pump inhibitors were able to control the symptoms, without increasing MTX.
After 3 years of stable moderate dysphagia (NRS 4/10), in 2015 postprandial vomiting relapsed and dysphagia worsened (NRS 7, 5/10) again, and the patient became unable to assume solid food.
Monthly IVIg (2 g/kg in 3 days) were started again, with a new quick improvement of dyspepsia, dysphagia and vomiting (NRS 5/10). After two months, Subcutaneous Immunoglobulins (SCIg), at the dose of 10 g twice a week, were proposed to increase the compliance of the patient (difficulty to reach the hospital). After 4 months she reported a further and remarkable improvement of symptoms (NRS 2, 5/10) and quality of life. The condition remained stable and satisfying during the 6 additional month of follow-up.
Dysphagia in the context of inflammatory muscle diseases, such as polymyositis or dermatomyositis and cancer associated myositis [4], is frequently described, with a highly variable prevalence of 30-60% [5,6].
Weakness of tongue, oropharyngeal and esophageal musculature, sometimes in the lower tract, can induce difficulties with swallowing. Patients report sensation of food sticking in the pharynx, coughing, choking, or regurgitation while eating, and difficulty with solid and dry foods. Dysphagia can sometimes be associated to several morbidities, including malnourishment, dehydration, social isolation, respiratory infections due to aspiration, and mortality [6].
Beyond symptomatic therapies, such as prokinetics, immunosuppressive therapy is the main treatment of PM related dysphagia. In particular, glucocorticoids are the mainstay of initial treatment of dysphagia. The addition of immunosuppressive drugs, such as methotrexate, azathioprine, mycophenolate mofetil and cyclosporine, is often required to control refractory disease, repeated disease flares, or to reduce the dose of corticosteroid therapy and associated side effects [2,7].
Data from uncontrolled studies in PM, indeed, documented the benefit of IVIg: this treatment is considered a second line therapeutic option, especially in patients with disease refractory to glucocorticoids and/or immune suppressants. Moreover, IVIg seems to achieve disease control in patients with severe muscle weakness (i.e. dropped head syndrome) or dysphagia [2,8-11].
To date, few data are available to evaluate IVIg on esophageal involvement in refractory PM; usually the regimen applied is 2 g/kg over 2-5 days. In 2002, Cherin, et al. carried out an open prospective study to evaluate the efficacy of IVIg in 35 subjects with PM that was refractory to traditional treatments. They administered IVIg in a regimen of 2 g/kg over 2 days monthly for mean 4-6 months. Eleven subjects presented esophageal disorders (including dysphagia, regurgitation and gastroesophageal reflux) evaluated with clinical assessment and manometry. In 8/11 (73%) patients they observed the complete disappearance of esophageal disorder [12].
In 2010, Marie, et al. retrospectively reviewed the medical records of 73 patients (39 with PM, 34 with DM) with steroid-resistant esophageal involvement, including dysphagia and gastroesophageal reflux. Patients were treated with IVIg (2 g/kg over 2 days monthly for a mean of 7 months) and 60/73 (82%) patients showed a rapid and significant improvement in esophageal symptoms [13].
In 2012, Miyasaka, et al. presented the results of a double-blind, placebo-controlled, crossover study designed to evaluate the efficacy and safety of polyethylene glycol-treated human IgG in 26 patients with corticosteroid-resistant PM (16 patients) or DM (10 patients). Nine patients presented dysphagia. They administered IVIg in a regimen of 2 g/kg over 5 days. Among 7 patients with esophageal involvement in the treatment group, 5 showed a disappearance of the symptoms, while no changes were observed in the 2 remaining subjects in the placebo group [14].
SCIg are a new therapeutic opportunity, which offers many advantages compared to IVIg, representing a safe alternative for difficult venous access, usually well accepted by patients [2,15,16]. SCIg have been usefully employed in various immune-mediated disorders, such as chronic inflammatory demyelinating neuropathy, multifocal motor neuropathy, other than primary and acquired immunodeficiency [15,17,18].
Pars K, et al. in 2013 reported a dramatic improve of dysphagia with IVIg in a 70-year-old patient affected by Inclusion Body Myositis (IBM). Then, the patient switched to SCIg (0.77 g/kg/month) and swallowing and body weight was constant over 4 years of follow up, preventing parental nutrition [19]. Also Cherin P, et al. reported 6 cases regarding patients affected by IBM describing a resolution of dysphagia with SCIg treatment [20]. In a following study, the same authors described 19 patients affected by inflammatory myophaties, among which 10 affected by PM or DM, successfully treated with SCIg, but no data were reported about dysphagia [21].
Danieli also described 11 patients affected by PM treated with SCIg with different administration schedules, including 6 patients with dysphagia [22]. She reported an improvement of the clinical and laboratory features regarding weakness and CK levels in all patients, without describing the evolution of dysphagia [22,23].
Finally, in 2014, Cherin reported the case of a female patient, 49-years-old, presenting a refractory PM with increasing dysphagia, and subsequent significant weight loss. After the failure of steroids and immune suppressive drugs and after the discontinuation of IVIg therapy because of problematic venous access, SCIg was given twice per week (2 then 1.3 g/kg/month). Within 2 months, a progressive improvement in muscle strength and the normalization of serum CK were observed. Dysphagia was also resolved, preventing parenteral nutrition and allowing for normal daily activities [24].
Up to date, this is the second observation describing an improvement of dysphagia in a patient affected by PM treated with SCIg. This therapy has demonstrated to be safe, well accepted and this could be a reasonable therapeutic option also in patient with refractory dysphagia in the context of inflammatory muscle diseases, such as PM.
Further studies are needed to evaluate the effectiveness of SCIg in the management of these patients.