A 73-year-old woman underwent total gastrectomy for cancer. Postoperative course was uneventful until day five when she felt unwell, showing distended and tender abdomen. Developed acute kidney injury and metabolic acidosis with a lactate level of 13.5 mmol/L. Given the strong suspicion of anastomotic failure, she underwent a urgent CT-scan that showed clear signs of diffuse bowel necrosis with evident pneumatosis intestinalis, along with gas both in venous mesenteric vessels and the liver. She was brought back to theatre night-time were almost the entire small bowel with most of the large bowel was resected. She deteriorated and died the day after. The acute mesenteric ischaemia was totally unexpected and unpredictable. In spite of our prophylactic measures, prompt diagnosis and surgical treatment, unfortunately, when the syndrome manifests, it is already unstoppable.
Acute mesenteric ischaemia, Pneumatosis intestinalis, Portomesenteric gas
The Acute Mesenteric Ischaemia (AMI) is a rare syndrome that develops as the consequence of a sudden decline in blood flow through the mesenteric vessels, due to occlusive disease or severe hypoperfusion. The events rapidly advance from ischaemia to necrosis of both small and large bowel that leads unavoidably to sepsis and death [1].
Unfortunately, the time window useful to diagnose and treat the condition is very narrow and symptoms are never specific, often misleading or absent, at least until the very last stage, when the damage becomes critical and irreversible. Thus, mortality rates have been remaining high for decades, still as high as 80%. Laboratory tests are non-specific and do not have a strong diagnostic impact. Computed tomographic angiography remains the preferred imaging test [2].
The key of success should be the early diagnosis, before intestinal infarction has occurred. Prompt surgical exploration with revascularization is required to avoid the spread of necrosis, saving intestinal integrity and resecting necrotic bowel segments [3].
We present a case of a patient operated on with total gastrectomy for cancer, who faced a lethal AMI at fifth postoperative day. The importance of early diagnosis, imaging specificity and prompt treatment are analysed and discussed.
A 73-year-old female presented to our outpatient clinic referred by her GP complaining of unexplained anaemia. On direct questioning, she noticed a darker colour of her stool and increased wind, but no change in bowel habit. She referred abdominal discomfort but no weight loss. The patient had a background of hypertension, hypothyroidism, chronic back pain, depression and bilateral hearing impairment. Her BMI was 24.7 and had no known history of drug allergy. Exercise tolerance was 500 yds.
Gastroscopy showed a middle gastric body cancer on the lesser curvature affecting 50% of the circumference. At CT-chest-abdomen-pelvis two conspicuous lower Para-oesophageal lymph nodes were identified but no evidence of distant metastases (TxN1M0). Gastric biopsy confirmed poorly-differentiated adenocarcinoma. At staging laparoscopy neither ascites nor distant metastases were observed.
The patient underwent three neoadjuvant chemotherapy cycles and, after that, repeated staging CT-scan which turned negative. Surgery was at that moment scheduled. During cardiologic pre-assessment, echocardiography showed an overall preserved systolic function with a left ventricular ejection fraction of 70%.
A total gastrectomy was accomplished without complications. Gastro-oesophageal junction and small bowel were transected, the last at 30 cm distally to duodenojejunal flexure. The alimentary loop was brought up for a retrocolic oesophago-jejunal anastomosis. The jejuno-jejunal anastomosis between the biliary and the alimentary limb was fashioned at 40 cm from the oesophago-jejunal one. A feeding jejunostomy was created at the end.
The patient was kept in the intensive treatment unit as routine precaution. Three more doses of antibiotics were prescribed. Given that haemoglobin level remained stable, she had prophylactic dalteparin 2500 IU eight hours post-op followed by 5000 IU every 24 hours. She started mobilisation and chest physio the day after surgery and stepped down to the ward after only 24 hours of intensive care.
The postoperative course proceeded uneventfully from day one to four. The pain was under control with no opioid analgesia required. She passed gas and enteral feeding, via jejunostomy, was started and tolerated. The patient was allowed to drink clear fluids. Complaints started on the evening of the fifth day post-operative, with abdominal distension and sporadic pain. She had no urine output, was hypotensive. Blood test revealed sudden increase in white cell count to 23.86 × 10^9/L (n.v.3.0-10.0), and CRP to 121.6 mg/L (n.v. up to 5.0). Developed acute kidney injury with rise in creatinine up to 123 µmol/L (n.v.49-92) and drop of GFR to 39 mL/min/1.73 sqm. Phosphate was also high: 2.44 mmol/L (n.v.0.87-1.45). Haemoglobin level remained otherwise stable at 120 g/L (n.v.115-175).
Patient was alert but looked unwell. Glasgow Come Scale was 15. She had nausea but did not vomit. The abdomen was distended with no bowel sounds, overall tender. Saturation was 92% with 2 L of O2. Temperature was 36.8 ℃, heart rate 75, respiratory rate 24, blood pressure 77/55, corresponding to a National Early Warning Score of 8: high risk. Arterial blood gas showed severe metabolic acidosis with pH: 7.132; PCO2: 5.69 kPa; HCO3: 13.9 mmol/L; BE: -14.4 mmol/L and lactate: 13.5 mmol/L. This deteriorating scenario was at first in keeping with intra-abdominal sepsis likely caused by anastomotic leak. Therefore, the patient was stepped up again to intensive care unit and underwent a urgent CT-abdomen.
Apart from air-fluid levels, the main finding was an extensive intramural and submucosal gas throughout most of the small bowel and right hemicolon. Gas was also localized within the mesenteric/portal veins and liver. This picture marked, hence, a profound small and large bowel ischaemia with established necrosis, involving the territory served by the superior mesenteric artery, in keeping with arterial obstructive mesenteric ischaemia (Figure 1).
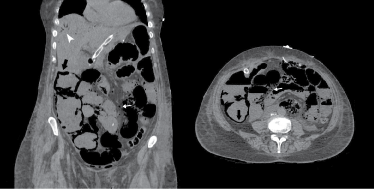 Figure 1: Abdomen CT-scan showing evidence of pneumatosis intestinalis (black arrow), mesenteric venous gas (white arrow) and gas in the liver (white arrowhead), specific picture for established bowel necrosis. View Figure 1
Figure 1: Abdomen CT-scan showing evidence of pneumatosis intestinalis (black arrow), mesenteric venous gas (white arrow) and gas in the liver (white arrowhead), specific picture for established bowel necrosis. View Figure 1
The patient was taken back to theatre for a urgent laparotomy. There was ischaemic intestine from jejuno-jenunostomy, involving the entire small bowel, to distal descending colon. There were only 20 cm of viable alimentary limb and 15 cm of viable biliary limb. The two anastomoses were intact with no evidence of leak. An internal hernia was found at the mesenteric gap between jejunal loops but it did appear of very little relevance. A massive intestinal resection was thereby performed. A decompressive intestinal stoma was fashioned on the biliary tract, whereas a nasogastric tube was left inside the alimentary limb also for decompression purpose. The colic stump instead was kept free intraperitoneally.
The specimens displayed extensive mucosal and submucosal infarction on both colon and small bowel. Thrombi were noted in the vessels in the region of the infarcts but most likely secondary to the infarction itself. In addition, there was submucosal fibrosis, suggesting an acute on chronic process. Eventually, there was no evidence of vasculitis.
After re-laparotomy the patient remained hypotensive and anuric. She was intubated and ventilated, commenced on vasopressors and started on hemofiltration. However, she continued to deteriorate. She failed to maintain saturation. She also developed multi organ failure, remained haemodynamically unstable and anuric. Following discussion with her family, it was decided not to further escalate treatment. She died on postoperative day seven.
The relevant etiopathogenesis of AMI along with clinical signs, laboratory and imaging tests, and above all the importance of an early diagnosis associated with a prompt surgical treatment will be gradually clarified.
Acute mesenteric ischaemia is defined as drop or frank occlusion of arterial or venous mesenteric blood flow that becomes insufficient to meet the demand. This rare condition accounts for 1:1000 of all the acute admission in USA and Europe. This incidence seems to increase exponentially in aged patient with a peak of incidence in the sixth and seventh decades, and an equal distribution between women and men [1].
Intestinal ischaemia may present as chronic or acute phenomenon. Chronic gastrointestinal ischaemia is most commonly due to atherosclerotic disease. In contrast, AMI represents the consequence of a rapid reduction in intestinal blood flow. It can be caused by either vascular arterial occlusion, both embolic (40-50%) and thrombotic (25-30%) or venous thrombotic occlusion (10%), or severe splanchnic hypoperfusion (20%). This last-mentioned Non-Occlusive Mesenteric Ischaemia (NOMI) is observed in patients undergoing major surgery and in patients with trauma, shock or sepsis [2,4,5]. In the presented case, despite a total gastrectomy carries an increased risk of NOMI, the syndrome developed after five days from major surgery, and appeared to be caused by the thrombotic obstruction of superior mesenteric artery, as it was in the end depicted at histopathology, in keeping with an acute thrombosis on a chronic scenario of severe atherosclerosis. The internal hernia discovered at re-laparotomy could have explained alone the whole picture, as the consequence of strangulation of the bowel inside the defect along with the subsequent venous thrombosis, but the size of this finding was not sufficiently relevant for being referred as the cause of the complication. In literature, the AMI due to arterial thrombosis carries a perioperative mortality ranging between 70 and 100% with and extent of ischaemia/infarct much greater than in case of embolic occlusion [5].
The diagnostic process still lacks in specific tests that may allow an early recognition. This happens because in the early stages only few unspecific clinical signs are present and they do not become evident until intestinal infarction has already occurred. Microscopic changes of bowel ischaemia can be detected within minutes. The gut cannot survive more than six hours of complete vascular occlusion. At that point, irreversible bowel injury occurs. Untreated, AMI will trigger the sequence of events that passes through mesenteric infarction, intestinal necrosis, sepsis and, eventually, death. An early recognition and a prompt intervention may halt and reverse this process optimizing recovery, whereas a late diagnosis, done after intestinal necrosis has developed, is the main responsible for the high mortality of the disease [1,3,4].
In the early phases of the mesenteric ischaemia, patients may complain of sudden onset of central abdominal pain, frequently colicky in nature, associated with vomiting. The concurrent sympathetic activation very often becomes evident with cold sweating. Nevertheless, the abdominal examination frequently shows minimal or unspecific signs until the bowel infarction reveals itself with all the very evident picture of peritonitis. Bowel sounds are usually hyperactive at the onset of ischaemia but silenced when infarct occurs. Patients generally did not show any fever. Faecal occult blood will not be demonstrated till mucosal infarction turns it positive. The subsequent loss of fluids into the intestinal lumen causes hypotension until real shock. Moreover, the same intestinal necrosis will push intestinal bacteria to translocate from bowel lumen to systemic blood flow, giving origin, in the end, to a septic shock [3-5].
It has been demonstrated that, because the small bowel is rich in phosphate, in 80% of patients with mesenteric infarction, the level of serum phosphate is significantly high. In addition, an elevation of serum lactate level at blood gas test indicates anaerobic metabolism and therefore, in the setting of bowel infarction, it is considered a very accurate test in the diagnosis of small bowel arterial ischaemia [6,7]. Nevertheless, lactic acidosis indicates at least segmental, but irreversible, bowel injury. Therefore, ideally, intervention would occur before evidence of increasing serum lactate level [3]. In our case, the triad: metabolic acidosis, leukocytosis and hyperphosphatemia was classically present, and in addition, lactate level was markedly elevated.
So far, laboratory abnormalities are not sufficiently sensitive or specific to be diagnostic. They are usually late signs, associated with bowel infarction. Novel serological biomarkers reflecting intestinal mucosal damage, i.e. Intestinal Fatty Acid-Binding Protein (I-FABP), α-Glutathione S-Transferases (α-GST) and Ischaemia Modified Albumin (IMA) seem promising. However, further investigations are required to specify threshold values and accuracy in early diagnosis of AMI [4,5,8].
CT-angiography represents the imaging test of choice with 96-100% sensitivity and 89-94% specificity. X-ray, ultrasound and magnetic resonance imaging have very limited indications in diagnosing AMI [2]. The presence of gas produced by bacteria in the intestinal wall is a very common finding at CT-scan. Moreover, the transmigration of the same bacteria from the bowel into the systemic circulation appears in the form of gas in the portomesenteric veins and inside the liver itself (Figure 1). Pneumatosis intestinalis along with portomesenteric venous gas are identified to be caused by bowel ischaemia in more than 70% of cases. When pneumatosis intestinalis is identified, together with portomesenteric gas, it is always an alerting sign. In those cases, the diagnostic specificity approaches approximately 100% for ischaemic bowel [6,9,10].
Initial medical treatment focuses on fluid resuscitation and correction of electrolyte imbalance, aiming at preventing multi-organ failure [3]. The shorter the duration of symptoms, the more likely is the patient to survive after surgery. However, surgery is almost always very aggressive. The removal of long segments of small bowel results in short bowel syndrome and intestinal failure, along with poor quality of life and increased mortality. Thus, extensive resections should be carefully considered in terms of real survival benefits. The goal of the surgical therapy of AMI is revascularization of the occluded vessel, by performing either embolectomy or arterial by-pass, and resection of necrotic/non-viable bowel. Surgery must proceed with minimal delay [1,3].
The reason why AMI has been remaining feared over decades lies in its unpredictability. Furthermore, clinical suspicion cannot rise as long as ischaemia has not turned into an irreversible infarct. Indeed, in the presented case, from the beginning of the symptoms to the diagnosis of AMI, only few hours had passed, but at that time necrosis had already spread. Given that the patient was recovering unremarkably, there was no reason at all to suspect a bowel ischaemia. Unfortunately, in spite of prophylaxis, prompt diagnosis and immediate surgical treatment, mortality is often clearly unavoidable.
No competing financial interests exist.