LPD: Luteal Phase Deficiency; CL: Corpus Luteum; LDL: Low Density Lipoprotein; BBT: Basal Body Temperature
Luteal Phase Deficiency (LPD), also known as luteal phase defect, is a concept that was defined by Georgeanna Seegar Jones in 1949 as reduced progesterone production by the Corpus Luteum (CL) [1]. LPD results from low endogenous progesterone production and the resultant insufficiency to maintain a secretory endometrium to allow embryo implantation and growth [2]. Jones studied the luteal phase by evaluating basal body temperature charts, urinary pregnanediol excretion (48-hour urine collection at the peak of luteal phase by basal body temperature), cervical mucus, and most importantly, timed endometrial biopsies. In the initial study, Jones evaluated 255 cycles in 98 women with infertility that was not due to tubal, uterine, anovulation or male factor. By assessing the delayed dating of the luteal phase endometrium, she determined that at least 50 percent of these women had either inadequate luteal stimulation or defective endometrial response, indicating many women could be suffering from this condition [3]. Noyes, a few years later, evaluated close to 400 histology slides of 100 uteri removed, to adequately date the histologic evaluation of endometrial biopsies [4] (Figure 1).
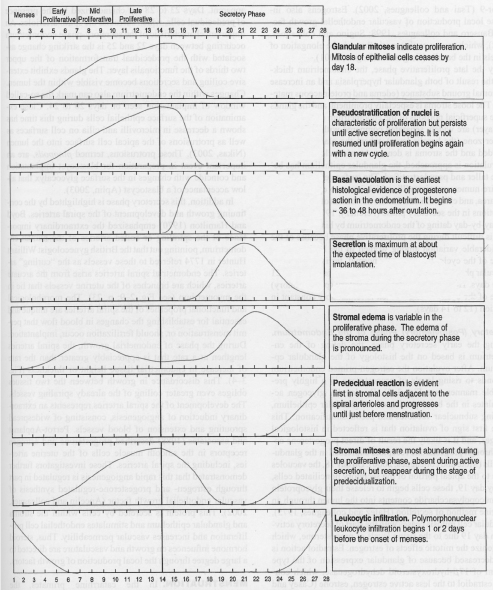 Figure 1: Curves representing estimated quantitative changes most helpful in dating the endometrium [13]. View Figure 1
Figure 1: Curves representing estimated quantitative changes most helpful in dating the endometrium [13]. View Figure 1
Luteal phase deficiency has been a controversial subject, with many doubting its existence and its association with infertility. The goal of this article is to review the historical and current understanding of LPD and its association with infertility.
The normal menstrual cycle includes a follicular phase and a luteal phase, which results in regular cycles and allows conception to occur. During the follicular phase, a mature dominant follicle develops, producing estrogen, which stimulates development of a proliferative endometrium. The end of the follicular phase occurs with maturation of the dominant follicle, and the LH surge, which is released from the anterior pituitary. This is propagated by increasing levels of estradiol that is produced from the granulosa cells of the dominant follicle [5]. After ovulation, the post-ovulatory follicle becomes the corpus luteum, producing progesterone from the granulosa-luteal cells of the corpus luteum. The progesterone released, stimulates and forms a secretory endometrium to prepare the endometrial lining for blastocyst implantation [5,6]. Maintenance and regulation of progesterone secretion is dependent on many factors including LH, presence of LH receptors, specific steroidogenic enzymes of the different cellular compartments of the ovary, and the amount and availability of substrate, namely cholesterol [7].
Cholesterol, in the form of Low Density Lipoprotein (LDL), is the primary precursor for progesterone. It also acts as a substrate for all other endocrine organs including the placenta, ovaries, testes, and adrenals [7]. Carr, et al. observed the maximal synthesis of progesterone by human corpus luteum tissue in culture was in the presence of both LDL and HCG, and establishing that LDL, rather than other sources of cholesterol, was critical [8] (Figure 2). In addition, Simpson, et al. demonstrated limited quantities of progesterone synthesis in pre-ovulatory human granulosa cells likely due to the absence of LDL, or cholesterol precursor, in the follicular fluid of women [9]. As seen in (Figure 3), the basement membrane of the follicle prevents LDL cholesterol from reaching the granulosa cells. After ovulation, the vessels invade the follicle and deliver LDL cholesterol to the granulosa-luteal cells. This allows for the enormous production of progesterone by the corpus luteum. Together, these observations support the hypothesis that progesterone production in the corpus luteum is dependent on LDL.
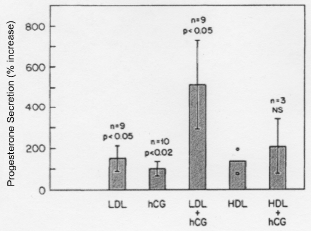 Figure 2: Progesterone secretion values of human corpus luteum tissue in the presence of LDL, hCG, LDL + hCG, HLD, and HDL + hCG [8]. View Figure 2
Figure 2: Progesterone secretion values of human corpus luteum tissue in the presence of LDL, hCG, LDL + hCG, HLD, and HDL + hCG [8]. View Figure 2
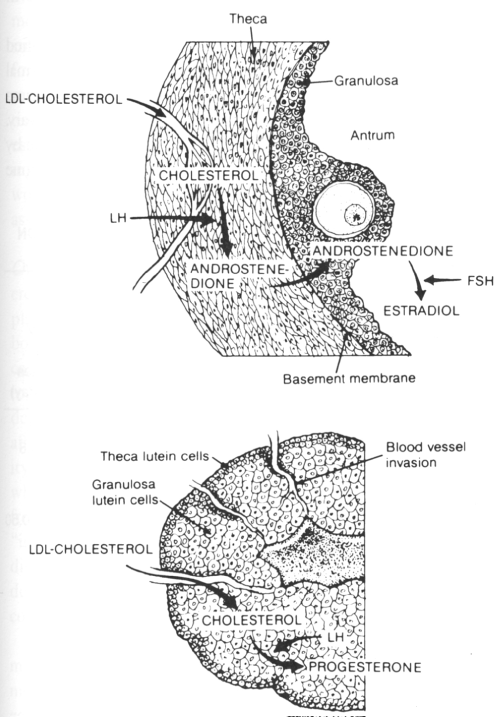 Figure 3: Figure demonstrating the regulation of progesterone secretion in the follicular phase and luteal phase of the human ovary [33]. View Figure 3
Figure 3: Figure demonstrating the regulation of progesterone secretion in the follicular phase and luteal phase of the human ovary [33]. View Figure 3
Originally, Csapo, et al. determined that the removal of the corpus luteum before 7 weeks of gestation resulted in loss of the pregnancy. After 7 weeks of gestation, the production of progesterone shifted from the corpus luteum to the placenta, and the pregnancy was maintained. Furthermore, the role of luteal progesterone in pregnancy has been studied extensively, and treatment with progesterone after luteectomy has demonstrated to positively affect pregnancy outcomes [10]. When progesterone therapy was supplemented after luteectomy at 6 weeks gestation, the pregnancy continued undisturbed [11]. Collectively, these examples demonstrate the vital role of progesterone synthesis from the corpus luteum in early pregnancy until the luteo-placental shift occurs [12].
The role of progesterone supplementation during In-vitro Fertilization and Frozen embryo transfers is the subject of several large and small studies but is beyond the scope of this review.
As described previously in 1950, Noyes, et al. defined the criteria involved in dating endometrial biopsy using a classic 28-day cycle, describing each morphological changes in detail (Figure 1). He believed the benefits of endometrial biopsies included the ability to quantitatively describe progesterone effects, giving a rough estimation of the time of ovulation [13]. Morphologically, there was no evidence of secretory vacuoles or changes that occurred until thirty-six to forty-eight hours after ovulation, and, therefore, secretory endometrium was not detected until day sixteen, or the second postovulatory day [13]. Endometrial histology evaluation during the secretory phase was believed to give more information regarding time of ovulation, integrity of the endometrium, and amount of progestational changes than any other test available at that time [13].
Clinicians continue to utilize endometrial biopsy during the luteal phase for the diagnosis of anovulation and LPD as a cause of infertility. A randomized blinded prospective multicenter study of 847 fertile and infertile women recruited at 12 clinical sites participating in the National Institutes of Health-funded Reproductive Medicine Network, underwent daily urinary LH testing. After the detection of LH surge, the patients were randomized to mid or late luteal phase biopsies. The prevalence of out-of-phase biopsies (> 2 day difference between the histologic criteria and the luteal day) was indistinguishable between women from fertile and infertile couples in either the midluteal or late luteal phase. In fact, the incidence of out of phase biopsies were significantly lower in the infertile women compared to their fertile counterparts (32.7% vs. 42.2%, P = 0.0248) [14], corroborating further evidence that endometrial biopsy is an inaccurate diagnostic tool for infertility or luteal phase deficiency. Due to the inaccuracy of histological evaluation, the endometrial biopsy is no longer helpful in evaluating the presence or absence of LPD as a cause of infertility.
A follow up of the same study determined that 7% of women who reported a positive surge of LH, in fact, had proliferative endometrium on biopsy, suggesting that either the LH surge or that the histological evaluation was incorrect [15]. The results suggest that many patients who have previously been identified with luteal phase deficiency may have been misdiagnosed from false positive urine luteinizing hormone testing and the inaccuracy of endometrial biopsies for this diagnosis.
Additional studies invalidate endometrial histology as a reliable source to measure luteal function. A randomized trial at an academic medical center that included 29 ovulating patients, ages 18-35, was conducted by obtaining endometrial samples from women in a natural cycle and two experimentally modeled cycle groups - GnRH agonist and a fixed dose of transdermal estradiol. This was followed by randomization of 10 or 40 mg progesterone administration. This study observed that histological endometrial dating did not correlate with circulating progesterone concentrations validating that endometrial histology is not an accurate measurement of luteal function or endometrial receptivity [16].
Other methods of diagnosing LPD include Basal Body Temperature (BBT) charts, measuring progesterone levels, and documentation of a shorter luteal phase. BBT rise occurs when progesterone increases during the mid-luteal cycle, roughly a temperature elevation of 1° F for every approximate rise of 2.5 ng/mL of progesterone [17]. While this method is thought to have a high sensitivity for detecting ovulation, it does not accurately correlate with endometrial histology [17], and up to 22.1% of women with hormonally normal menstrual cycles may experience an abnormal BBT [18]. While measurement of progesterone levels can be obtained by the serum, urine, or saliva, there is no agreed level for normal progesterone in the midluteal phase. Also, the slow pulsing of LH during the luteal phase results in fluctuation of progesterone levels, making this single result a poor and unreliable marker [17]. Though the short luteal phase, thought to be a subset of LPD, has been associated with infertility in the past [19], Smith, et al. found no statistical difference in luteal phase length when comparing two populations of fertile and infertile women [20]. Another significant discrepancy found in prior studies was the variable dating cutoff, ranging anywhere from 8 days [21] to 11 days [20]. Review of these methods confirms the unreliable diagnostic tools available for the diagnosis of LPD.
While LPD has been associated with infertility in the past, the current American Society of Reproductive Medicine committee opinion released in 2012 regarding the clinical relevance of luteal phase deficiency, states there has been insufficient evidence presented to support LPD as an independent association with infertility [2]. The lack of LPD determined by endometrial biopsy in infertility has been notable, specifically in a population of 137 women diagnosed with and without LPD. They observed no significant difference in fertility outcomes [22]. Another retrospective study by Balasch, et al. included evaluation of endometrial biopsies of over 1000 patients, determining the diagnosis of LPD occurred as a chance event. 7 out of the 10 patients diagnosed with LPD by three endometrial biopsies in three separate cycles, achieved term pregnancies without progesterone treatment [23]. A more recent prospective time-to -pregnancy cohort study of 1,635 cycles, reported 18% of cycles with a short luteal phase (< /- 11 days) compared with women with a luteal phase of 14 days, and the results showed no significant increased incidence of infertility at 12 months [24]. The studies continue to validate the lack of association between LPD and infertility.
Illingworth, et al. reported the hormonal changes during the menstrual cycle in a 19-year-old woman with regular cycles who had deficient LDL cholesterol. In comparison to a control subject, she demonstrated normal estrogen secretion during the proliferative cycle and equal levels of FSH and LH surge, suggesting that ovulation occurred [25]. However, she had only slight elevation of progesterone secretion (1.5 ng/ml) in the luteal phase compared to a normal control subject (10 ng/ml) (Figure 4). This patient appeared to be a unique example of severe LPD. Even so, at age 22, she spontaneously conceived and delivered a healthy liveborn infant at term. While this patient had significant decreased levels of progesterone recorded throughout pregnancy, compared to normal controls, it raises the question of how much progesterone is required for a normal pregnancy [26] (Figure 5). It is noteworthy that this pregnancy occurred spontaneously and was carried to term without progesterone supplementation.
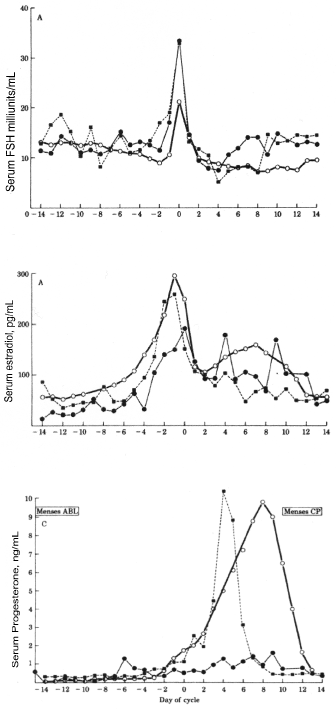 Figure 4: Serum concentrations of FSH, estradiol, and progesterone during the menstrual cycle in a patient with homozygous hypobetalipoproteinemia (ÿ), normolipidemic control (▪), and in normal women, [25]. View Figure 4
Figure 4: Serum concentrations of FSH, estradiol, and progesterone during the menstrual cycle in a patient with homozygous hypobetalipoproteinemia (ÿ), normolipidemic control (▪), and in normal women, [25]. View Figure 4
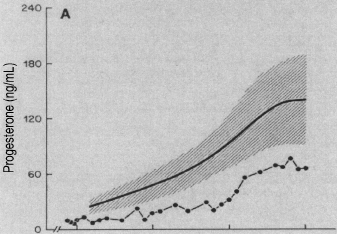 Figure 5: Serum levels of progesterone throughout pregnancy in a woman with homozygous hypobetalipoproteinemia. The solid circles represent values for the patient. The shaded area represent mean values ± 1 SD for normal women during gestation [26]. X axis represents weeks of gestation and each line represents 10 weeks of gestation (i.e. 10, 20, 30, 40 weeks of gestation). The Y axis represents Progesterone (ng/ml). View Figure 5
Figure 5: Serum levels of progesterone throughout pregnancy in a woman with homozygous hypobetalipoproteinemia. The solid circles represent values for the patient. The shaded area represent mean values ± 1 SD for normal women during gestation [26]. X axis represents weeks of gestation and each line represents 10 weeks of gestation (i.e. 10, 20, 30, 40 weeks of gestation). The Y axis represents Progesterone (ng/ml). View Figure 5
Limited studies have been conducted with luteal phase progesterone supplementation, and the few studies conducted have had little to no association with successful pregnancies [27]. Annos, et al. attempted diagnosis and treatment for LPD in 29 patients using BBT chart, endometrial biopsy, and progesterone levels. Of the 29 patients included in this study, 14 patients met study specific criteria for LPD, and at least 50% of all cycles showed discrepancy between the endometrial biopsy and progesterone level. In addition, the 14 patients that met criteria were randomized to treatment with progesterone vaginal suppositories, clomiphene citrate, or no treatment, and the results remained inconclusive. Though the results may have been influenced by the limited patient size, the author comments that the “treatment of LPD is as controversial as its diagnosis” [28].
Additionally, there are few randomized controlled trials, but rather most of the scientific evidence is based on review and meta-analyses of observational studies [29]. While most treatment is given experimentally in hopes of enhancing early pregnancy implantation, it is based on limited reliable data [2].
A newer method capable of diagnosing endometrial receptivity for implantation, irrespective of endometrial histology, is the Endometrial Receptivity Array (ERA)-a customized array of greater than 200 genes coupled to a computational predictor proficient in diagnosing a functional endometrium [30]. Evaluation of the endometrial receptivity has been helpful in patients with repeated implantation failure in order to better tailor timing of implantation with the endometrium [30]. A comparative prospective study of eighty-six healthy oocyte donors, demonstrated the endometrial receptivity array as a superior diagnostic method for detecting endometrial receptivity compared to histologic evaluation [31]. While the transcriptomic data seems promising for the future of fertility, Wang, et al. explains the need for further maturation of this technology prior to considering it the vanguard technology for clinical utilization as the natural and stimulated cycles differ significantly in their gene profiles of endometrial receptivity [32].
• After review of the historical and more recent research available regarding luteal phase deficiency, insufficient evidence exists to confirm the presence of luteal phase deficiency and its association with infertility.
• Endometrial biopsy as an evaluation for luteal phase deficiency should not be included in the work up of infertility alone.
• There is insufficient evidence to support the treatment of luteal phase deficiency with exogenous progesterone or exogenous hCG.