Chemotherapy-induced severe hyponatremia is a life-threatening condition. Platinum-based agents play a key role in ovarian cancer treatment, but they are more likely to cause hyponatremia than other anti-cancer agents. The optimal strategy for treating ovarian cancer in the case of severe hyponatremia induced by the platinum agent remains unclear. We report the case of 77-year-old women with multiple peritoneal recurrences five years and eight months after surgery for ovarian clear cell carcinoma. She received tri-weekly docetaxel and carboplatin (DC) therapy and bevacizumab, following which she developed severe hyponatremia due to syndrome of inappropriate antidiuretic hormone secretion (SIADH). We were reluctant to change the drugs because they effectively reduced the cancer antigen 125 level. The chemotherapy regimen was changed to weekly administration off a divided dose of DC, and she completed six cycles of weekly DC without electrolyte disturbance, including hyponatremia, or tumor recurrence. Weekly carboplatin administration may be a promising alternative to tri-weekly carboplatin administration after the development of carboplatin-induced SIADH.
Hyponatremia, Syndrome of inappropriate antidiuretic hormone secretion, Carboplatin, Divided dose
AUC: Area Under the Curve; CA125: Cancer Antigen 125; SIADH: Syndrome of Inappropriate Antidiuretic Hormone Secretion; RSWS: Renal Salt Wasting Syndrome
Hyponatremia occurs in 2.6%-29.1% of patients receiving chemotherapy [1]. Although platinum-based agents play a key role in ovarian cancer treatment, they are more likely to cause hyponatremia than non-platinum-based agents (incidence of hyponatremia: 11.9% and 3.8%, respectively) [1]. In previous reports, platinum-based drugs were often replaced with non-platinum-based drugs or other platinum-based drugs or discontinued due to severe drug-induced hyponatremia; however, the optimal strategy is still unknown. Here, we report a case of severe hyponatremia following tri-weekly carboplatin administration for treating recurrent ovarian clear cell carcinoma. We changed the regimen to weekly administration by dividing the dose of carboplatin, and no hyponatremia occurred despite not changing the drugs.
A 77-year-old woman underwent total abdominal hysterectomy, bilateral salpingo-oophorectomy, partial omentectomy, and pelvic and para-aortic lymphadenectomy for treating right ovarian cancer. The postoperative diagnosis was right ovarian clear cell carcinoma (stage IC3). She received adjuvant chemotherapy with paclitaxel (75 mg/m2) and carboplatin (area under the curve [AUC]: 2) on days 1, 8, and 15. On day eight, the regimen was discontinued because of elevated serum liver enzyme levels due to drug-induced liver injury (aspartate aminotransferase: 284 U/L, alanine aminotransferase: 344 U/L, and lactate dehydrogenase: 406 U/L). The regimen was modified to irinotecan (60 mg/m2 on days 1, 8, and 15) and cisplatin (60 mg/m2 on day one). However, on day six, she experienced convulsions and hyponatremia (serum sodium level: 109 mEq/L). She was treated with supplemental sodium chloride, and her sodium level recovered six days after the onset of convulsions. Hence, the patient was followed without adjuvant chemotherapy. Five years and eight months after the surgery, the patient's cancer antigen 125 (CA125) level was elevated to 569 U/L, and positron emission tomography/computed tomography showed multiple peritoneal dissemination, indicating recurrence of ovarian cancer (Figure 1). She received chemotherapy with docetaxel (70 mg/m2 on day one), carboplatin (AUC: 6 on day one), and bevacizumab (15 mg/kg on day one) because of her history of liver injury and hyponatremia following paclitaxel and cisplatin administration, respectively. On day five, the patient complained of general malaise. Hematological examination revealed a serum sodium level of 115 mEq/L, antidiuretic hormone level of 7.5 pg/mL, creatinine level of 0.52 mg/dL, and cortisol level of 31.5 μg/dL, with a plasma osmolality of 236 mOsm/kg, and urine osmolality of 631 mOsm/kg. Physical examination did not reveal any symptoms of hypovolemia or hypervolemia. She was diagnosed with carboplatin-induced syndrome of inappropriate antidiuretic hormone secretion (SIADH) and treated with hypertonic saline infusion. Her serum sodium level gradually rose to normal levels eight days after sodium supplementation (Figure 2). Although SIADH occurred, the CA125 level reduced to 66 U/L, demonstrating the efficacy of the regimen. The patient received chemotherapy with divided doses of docetaxel (35 mg/m2 on days 1, 8, and 15) and carboplatin (AUC: 2 on days 1, 8, 15), for which she provided informed consent. After weekly administration of docetaxel and carboplatin (weekly DC), her CA125 levels further decreased to normal levels. She completed six cycles of weekly DC without electrolyte disturbance or tumor recurrence.
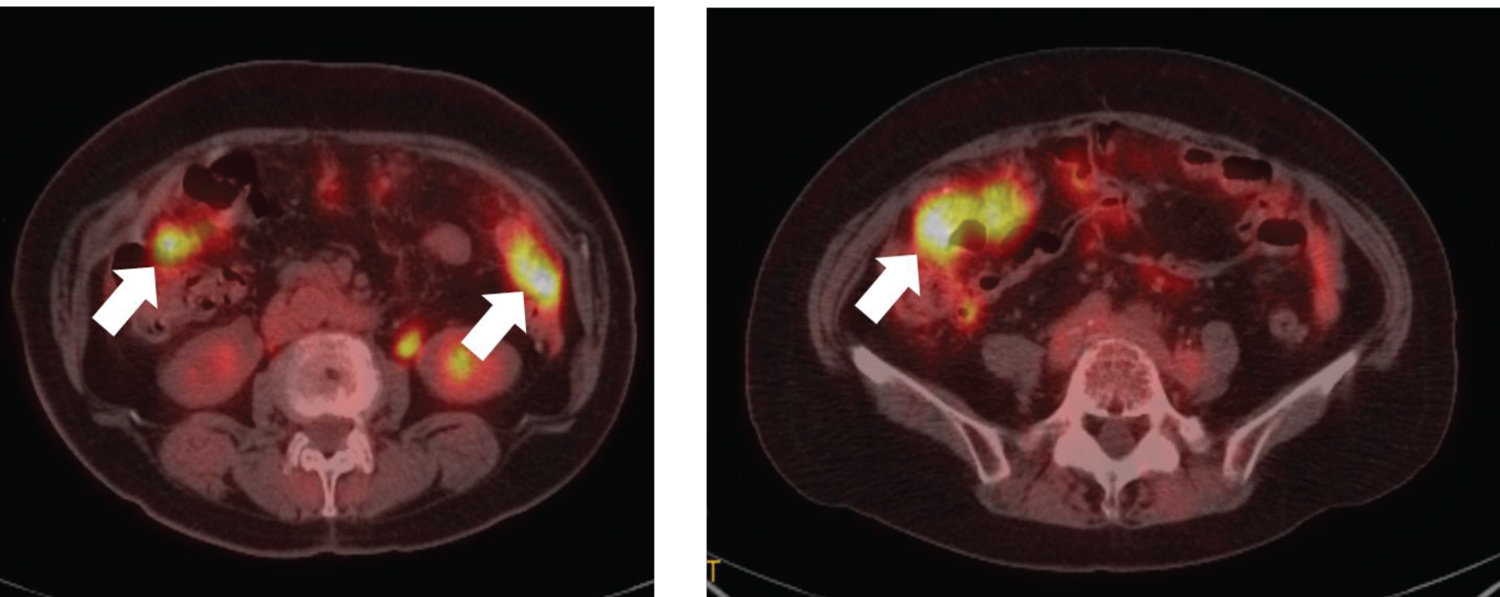 Figure 1: Positron emission tomography/computed tomography image showing multiple peritoneal dissemination of recurrent ovarian cancer (white arrows). View Figure 1
Figure 1: Positron emission tomography/computed tomography image showing multiple peritoneal dissemination of recurrent ovarian cancer (white arrows). View Figure 1
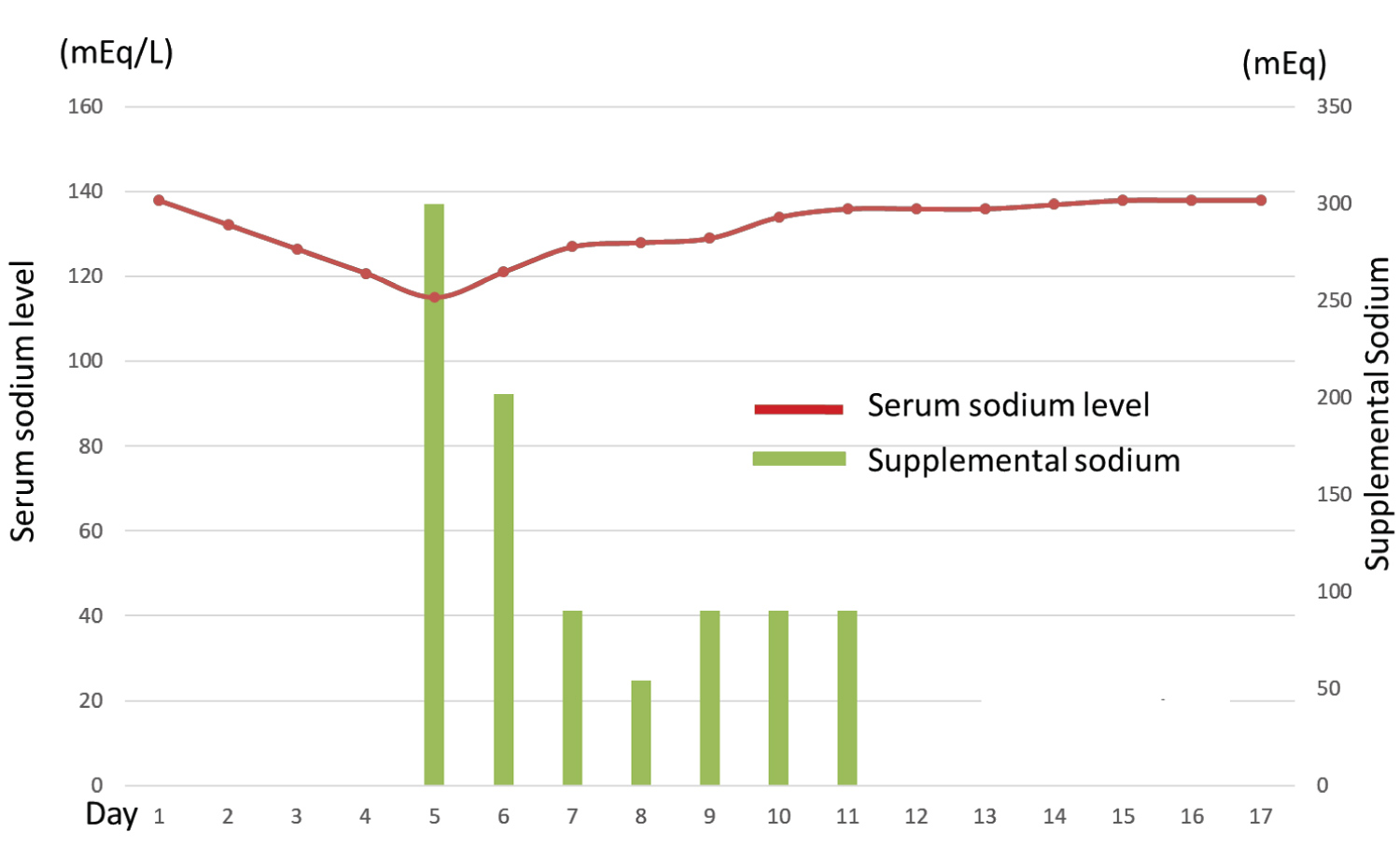 Figure 2: The patient's serum sodium level decreased to 115 mEq/mL on day five of chemotherapy. It was corrected with supplemental sodium, which was discontinued on day 21. Inappropriate ADH secretion occurred on day 5 of chemotherapy, but its serum level was within the normal range on day 15.
Figure 2: The patient's serum sodium level decreased to 115 mEq/mL on day five of chemotherapy. It was corrected with supplemental sodium, which was discontinued on day 21. Inappropriate ADH secretion occurred on day 5 of chemotherapy, but its serum level was within the normal range on day 15.
ADH: Antidiuretic hormone.
View Figure 2
Platinum-based agents have been known to cause SIADH and renal salt wasting syndrome (RSWS), leading to hyponatremia. SIADH is a disorder of impaired water excretion due to the over-secretion of ADH. Its treatment includes fluid restriction and sodium supplementation. The occurrence of SIADH has been associated with ectopic ADH-producing tumors, lung disease, surgical stress, central nervous system disease, and the use of certain medication, as was the case here [2]. In RSWS, impaired proximal tubular reabsorption of sodium and water induces hyponatremia and dehydration; it is often caused by platinum-based agents. The treatment of RSWS includes fluid replacement. Differential diagnosis is difficult but important because the treatment strategy differs [3].
Ezoe, et al. reported that cisplatin caused hyponatremia more often than carboplatin; the incidence of grade 3/4 hyponatremia was 13.5% in patients treated with cisplatin and 7.6% in those treated with carboplatin [1]. They attributed this difference to nephrotoxicity due to the mechanism of cisplatin excretion [4]. We have listed the previous reports of carboplatin-induced hyponatremia in Table 1. Only five cases of hyponatremia after carboplatin administration have been reported; among them, one patient had RSWS [5] and the other four had SIADH [6-9]. While RSWS is a common cause of cisplatin-induced hyponatremia [10], SIADH is the most common cause of carboplatin-induced hyponatremia.
Table 1: Reports of carboplatin-induced syndrome of inappropriate of antidiuretic hormone secretion. View Table 1
Our patient did not have dehydration or renal dysfunction, which indicated that SIADH was the most likely cause of the hyponatremia. Grade 4 hyponatremia occurred after the administration of docetaxel, carboplatin, and bevacizumab. There are no reports of bevacizumab-induced hyponatremia, and only one case of nab-paclitaxel-induced hyponatremia has been reported [11]. Our patient's SIADH might have been caused by the administration of carboplatin (AUC: 6). In previous reports, carboplatin administration was continued in only one of four patients who developed carboplatin-associated SIADH [9], and the drug was changed or discontinued in the other three [6-8]. The patient in whom carboplatin administration was continued had grade 3 hyponatremia, which recovered following oral sodium supplementation, and chemotherapy was discontinued after two cycles because the regimen was not effective [9].
In this case, we administered a divided dose of DC, allowing us to continue chemotherapy without reducing the total dose of carboplatin, which was found to be effective, and without the patient developing SIADH. Recurrent ovarian clear cell carcinoma is resistant to chemotherapy [12]; therefore, it is important to avoid discontinuing the use of platinum-based agents. The MITO-7 [13] and ICON-8 [14] trials found that a weekly regimen of carboplatin and paclitaxel was not significantly inferior to a tri-weekly regimen for the treatment of ovarian cancer. Although bevacizumab has been shown to be beneficial for the treatment of recurrent ovarian clear cell carcinoma [15], we did not administer bevacizumab to our patient because a regimen of bevacizumab combined with weekly DC therapy is not adopted at our hospital.
Weekly carboplatin administration may be a promising alternative to tri-weekly carboplatin administration after the development of carboplatin-induced SIADH.
The authors declare no conflicts of interest. We would like to thank Editage (https://www.editage.com/) for English language editing.
All authors contributed equally to this work.