Current recommendations for pregnant women with cervical cancer depend on several factors, such as the stage of the disease, the gestational age at the time of diagnosis, and the patient's desire to maintain the pregnancy. This report describes the case of a 24 weeks pregnant woman with stage IB2 cervical cancer who was successfully treated with three cycles of platinum-based neoadjuvant chemotherapy followed by a simultaneous Сaesarean section and radical hysterectomy. Her newborn daughter was completely healthy at birth. As far as we know, this was the first case of cervical cancer during pregnancy to be treated with this approach in Belarus. In the absence of clear guidelines, these patients should be treated after a decision by a multidisciplinary cancer committee, including a gynecologist-oncologist, obstetrician, pathologist and neonatologist, to determine the best treatment option.
Cervical cancer, Pregnancy, Neoadjuvant chemotherapy, Simultaneous caesarean section, Radical hysterectomy
Due to the current trend of having children at an older age, the number of pregnancies complicated by cervical cancer is increasing [1]. Radical hysterectomy with pelvic lymph node dissection is currently the standard of care for cervical cancer patients with International Federation of Gynecology and Obstetrics (FIGO) 2018 IA2-IB2 Stages [2,3]. In pregnant women this treatment is possible planning in cases of pregnancy no preserving management. But analysis of the prognosis of cervical cancer during pregnancy shows the absence of a negative impact of pregnancy on the patient's outcome [4-6]. Therefore, management with continued pregnancy should be considered initially. According to the recommendations of the International Network on Cancer, Infertility and Pregnancy (INCIP) after the 22nd week of gestation, delayed treatment after delivery with regular follow-up could be initiated. Alternatively, Neoadjuvant Chemotherapy (NACT) could be used to give time to achieve fetal viability by preventing disease progression [5,7]. However, data on pharmacokinetics of chemotherapy during pregnancy are scarce and current evidence is based on relatively small case series [5,8]. The purpose of this report was to evaluate the outcomes of neoadjuvant chemotherapy during pregnancy followed by Cesarean section and radical hysterectomy in a woman with cervical cancer who had her first pregnancy and had a strong interest in keeping it.
A 31-year-old nulliparous woman was referred to the NN Alexandrov National Cancer Centre at 24 weeks of pregnancy with unremarkable medical, surgical, or family history. During prenatal care at the time of 22 weeks' gestation she had presented with abnormal vaginal bleeding. Since the patient was pregnant for the first time, she refused to terminate the pregnancy.
Patient underwent extensive workup including gynecologic exam, colposcopy evaluation, pelvic Magnetic Resonance Imaging (MRI) and biopsy of the cervix.
On pelvic examination an exophytic cervical tumor measuring 3 cm in diameter on the posterior lip of the cervix was found; there were no evidence of parametrial or vaginal involvement. Colposcopy examination was performed, which confirmed the tumor on the posterior lip of the cervix (Figure 1).
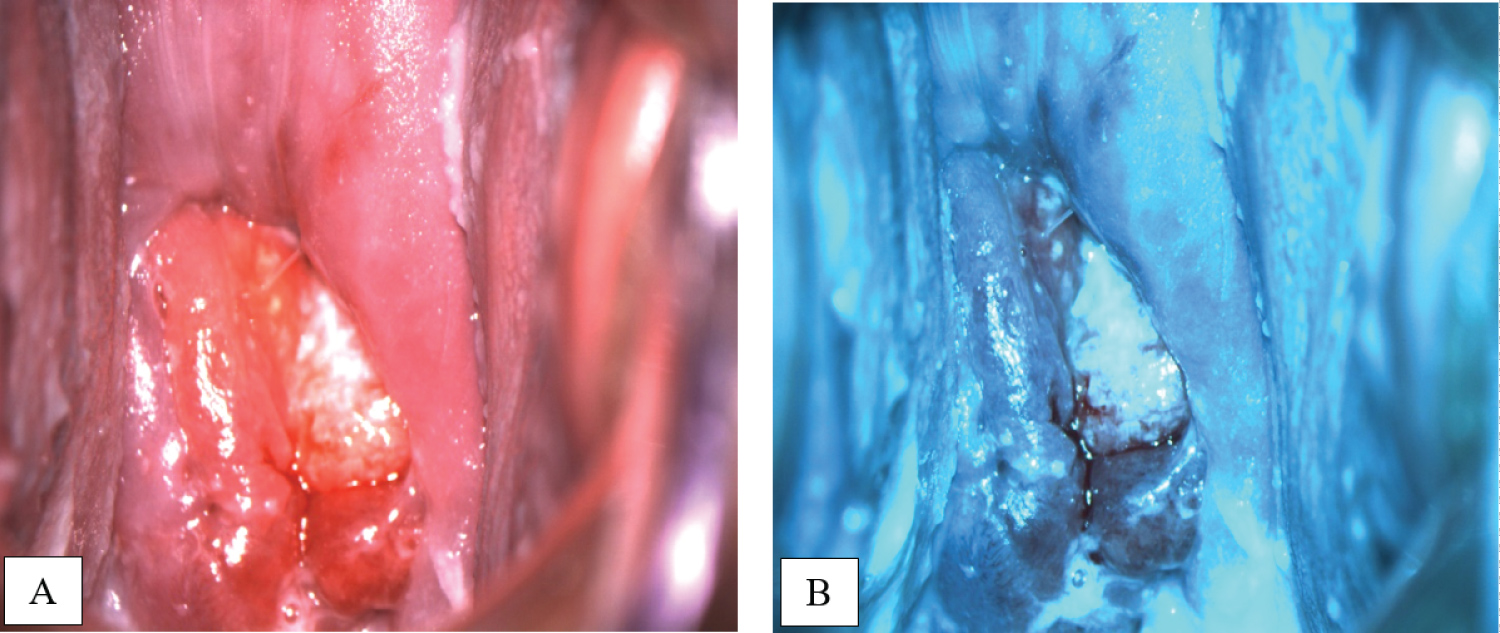 Figure 1: Colposcopy images in white (A) and green (B) filters show tumor on the posterior lip of the cervix. View Figure 1
Figure 1: Colposcopy images in white (A) and green (B) filters show tumor on the posterior lip of the cervix. View Figure 1
Staging studies with pelvic MRI allowed to visualize an intrauterine pregnancy and cervical mass measuring 1.7 by 2.5 by 3.0 cm, mainly on the back lip of the cervix; cervical ring was preserved and there were no suspicious pelvic or inguinal lymphadenopathy (Figure 2).
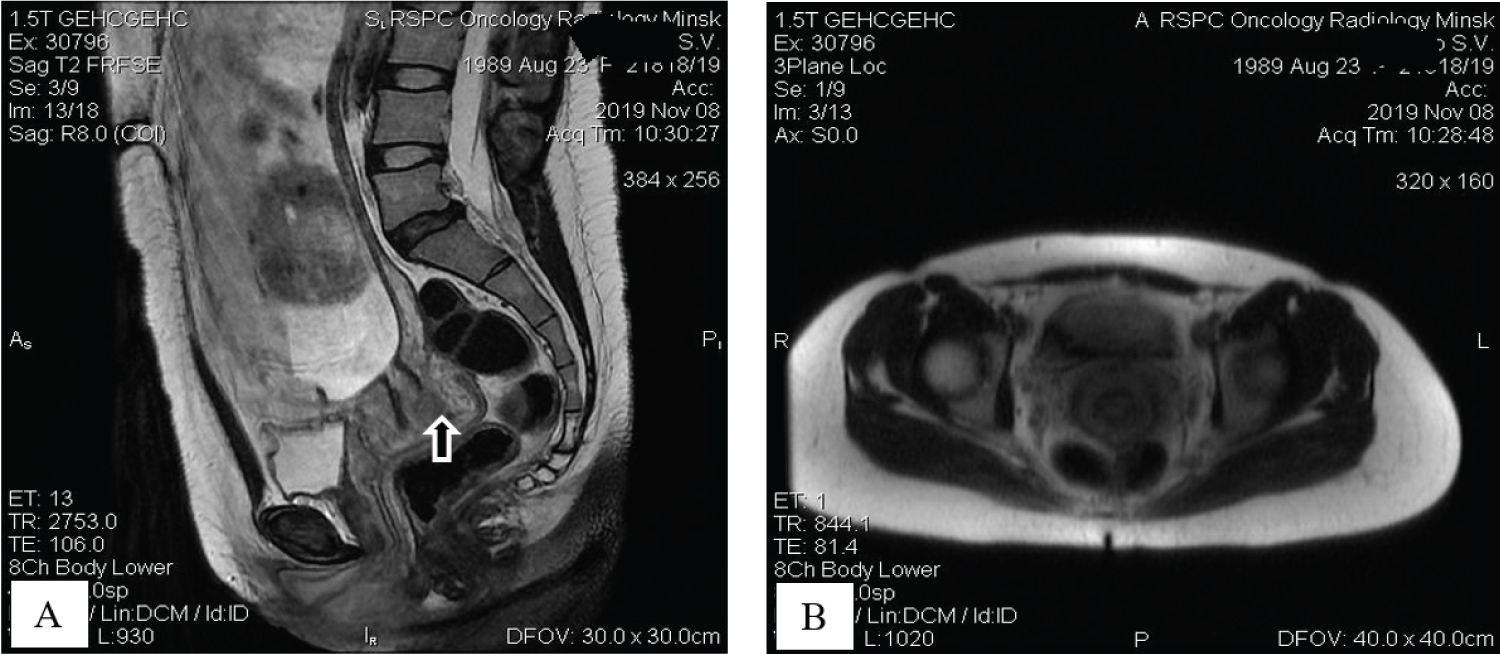 Figure 2: Tumor visualization with sagittal T2-weighted MR image demonstrates intrauterine gestation and cervical mass on the back lip of the cervix with preserved cervical ring (A, arrow); coronal T2W MR view shows no pelvic lymphadenopathy (B). View Figure 2
Figure 2: Tumor visualization with sagittal T2-weighted MR image demonstrates intrauterine gestation and cervical mass on the back lip of the cervix with preserved cervical ring (A, arrow); coronal T2W MR view shows no pelvic lymphadenopathy (B). View Figure 2
Histological examination of biopsy of the cervix revealed invasive squamous cell carcinoma, G2, without convincing evidence of lymphvascular space involvement (Figure 3).
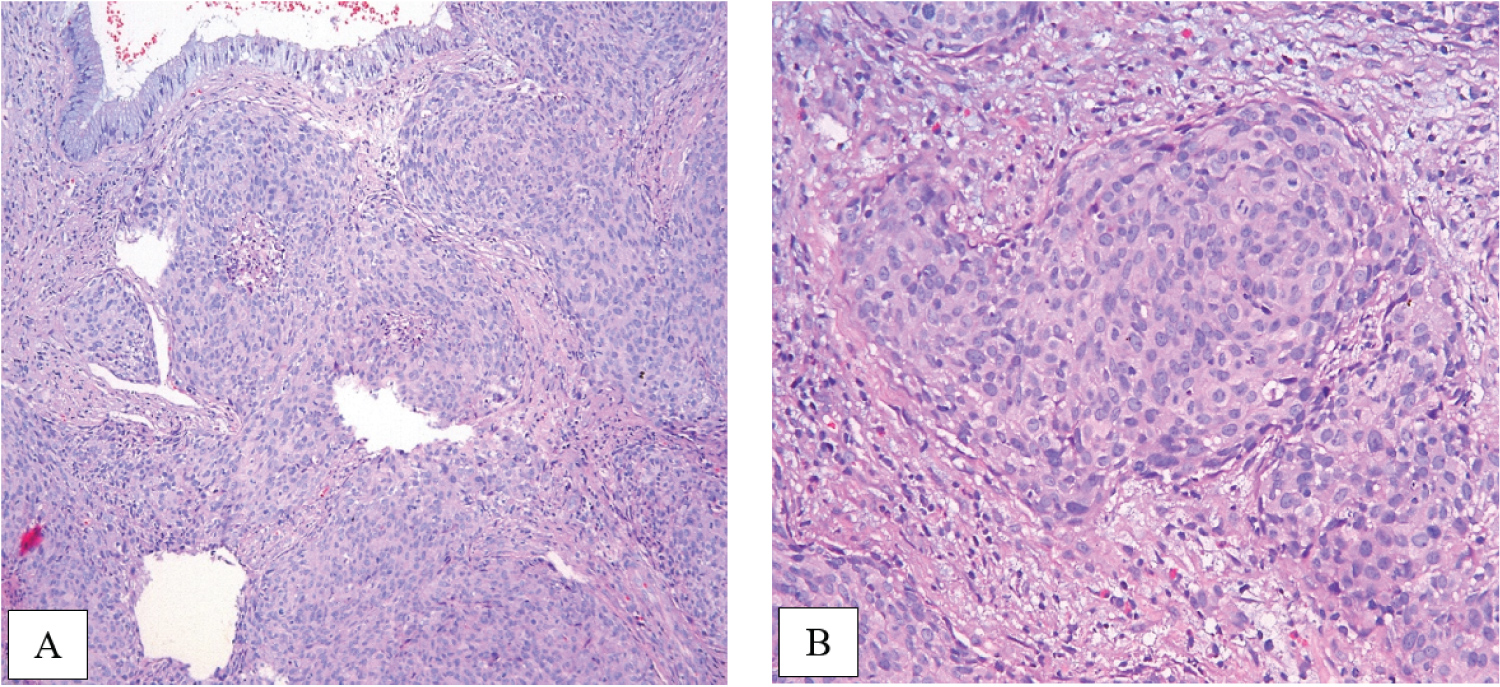 Figure 3: Morphology slides demonstrate invasive squamous cell carcinoma, grade 2 (A, × 10), (B, × 20). View Figure 3
Figure 3: Morphology slides demonstrate invasive squamous cell carcinoma, grade 2 (A, × 10), (B, × 20). View Figure 3
Based on these results and according to the 2018 FIGO classification, the patient was diagnosed with stage IB2 invasive squamous cell carcinoma of the cervix in combination with a 24-week pregnancy.
The patient's treatment plan was discussed during Multidisciplinary Conference, including a gynecologic oncologist, obstetrician, neonatologist, pathologist, and medical oncologist. Given the patient's strong desire to preserve the pregnancy, to help reach fetal maturity, which is defined as a delivery after 37 weeks' gestational age, platinum-based neoadjuvant chemotherapy was admitted.
Three courses of paclitaxel and carboplatin scheme were performed with 3-weekly interval. Patient tolerated chemotherapy well without any signs of toxicity. The pregnancy continued with no complications too. The efficacy of neoadjuvant chemotherapy was confirmed by colposcopy examination (Figure 4) and pelvic MRI 3 weeks after the completion of the third course of NACT.
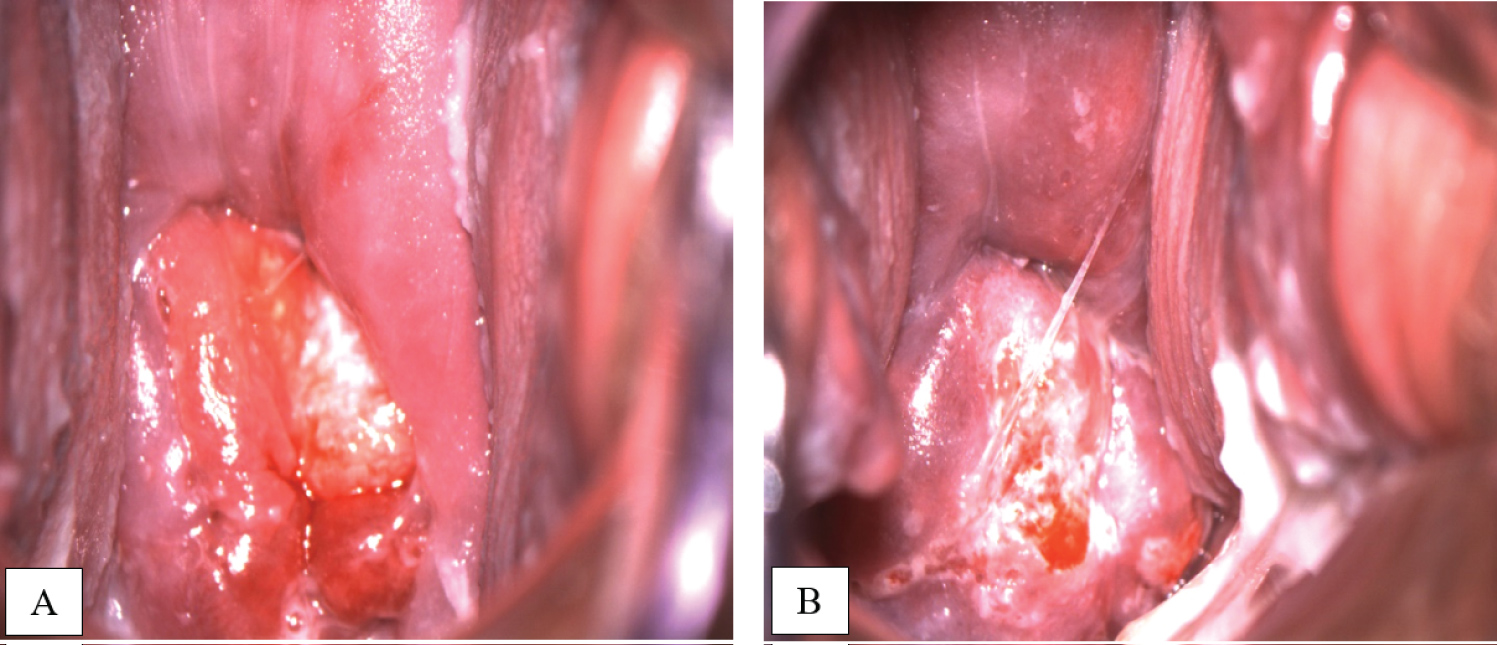 Figure 4: A) Colposcopy examination before NACT in 24 weeks gestation; B) After 3 courses of NACT in 37 weeks gestation. View Figure 4
Figure 4: A) Colposcopy examination before NACT in 24 weeks gestation; B) After 3 courses of NACT in 37 weeks gestation. View Figure 4
At 37 weeks' gestationa Cesarean section was performed using the lower midline skin incision. A female child was delivered. Birth weight of infant was 3380 grams, height - 52 cm; the Apgar score was 8.8 points. After delivery, a radical abdominal hysterectomy (type III, C) [9] was performed with pelvic lymph node dissection, bilateral tubectomy, and ovarian transposition. Macroscopically, there are no signs of a tumor of the cervix (Figure 5).
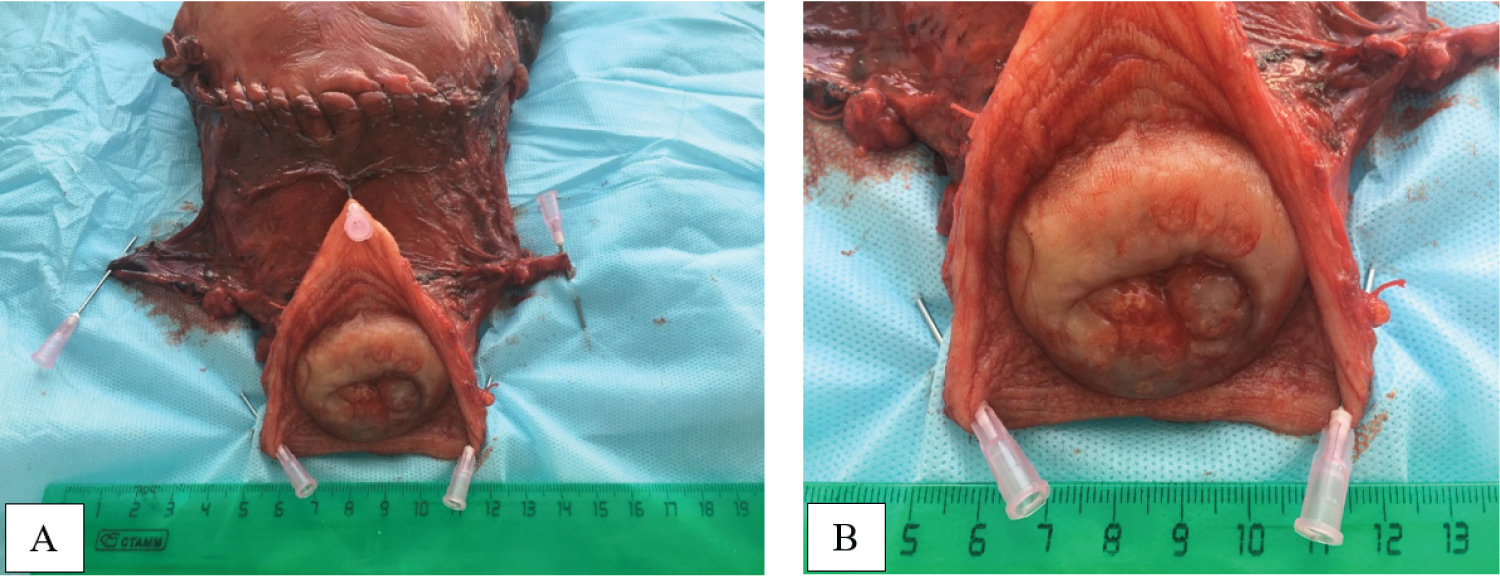 Figure 5: A macroscopic view of hysterectomy specimen with Cesarian scar, parametrial tissues and cervix with vaginal flap (A, B). View Figure 5
Figure 5: A macroscopic view of hysterectomy specimen with Cesarian scar, parametrial tissues and cervix with vaginal flap (A, B). View Figure 5
Final pathology report of hysterectomy specimen showed small evidence of invasive squamous cell carcinoma, grade 2, with depth of invasion 3.5 mm and horizontal spread 3.5 mm. But multifocal invasion of lymphatic vessels was determined. Endometrium with decidual transformation, residual chorionic villi in the area of the placental site were found. The underlying myometrium was with interstitial edema. The both fallopian tubes were of normal structure. Cutting edge of the vagina had no tumor growth. Pelvic lymph nodes (23) were negative, with foci of endosalpingiosis and subcortical islets of decidual tissue in some of them (Figure 6).
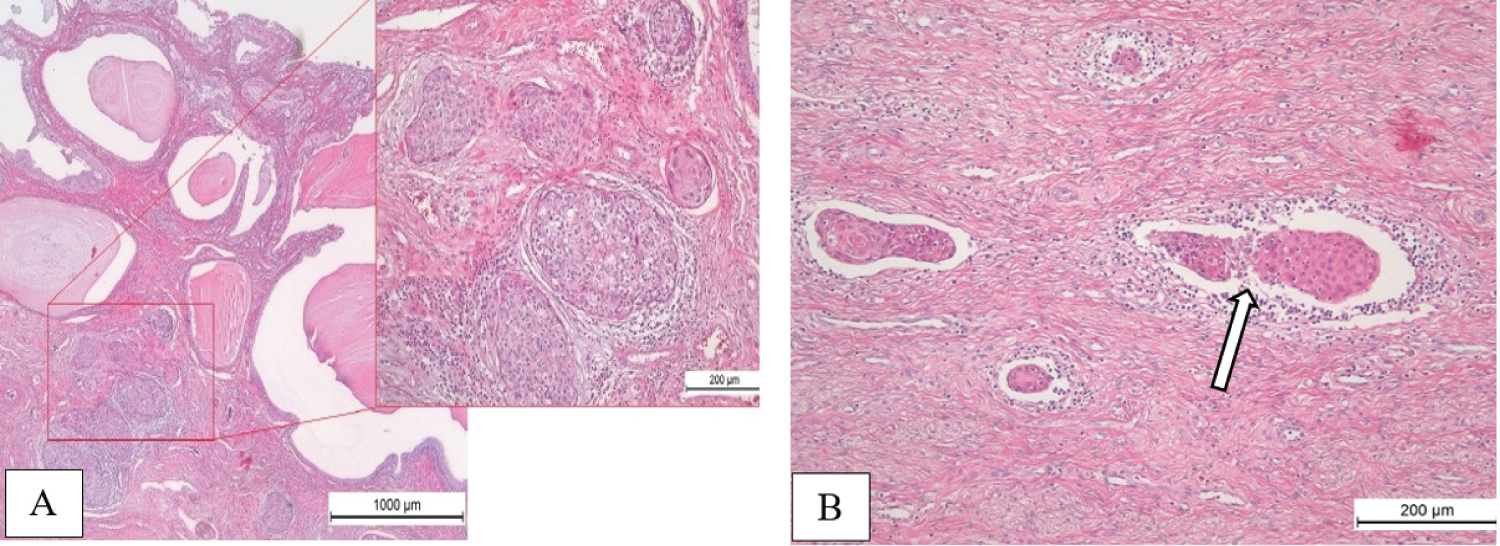 Figure 6: Pathology of hysterectomy specimen. Small focuses of invasive squamous cell carcinoma, depth of invasion 3.5 mm (A); Tumor emboli in lymphatic vessels (B, arrow). View Figure 6
Figure 6: Pathology of hysterectomy specimen. Small focuses of invasive squamous cell carcinoma, depth of invasion 3.5 mm (A); Tumor emboli in lymphatic vessels (B, arrow). View Figure 6
Based on these data and after Multidisciplinary Conference, postoperative course of radiotherapy was admitted. External beam pelvic radiotherapy at a total dose of 44 Gy fractionated over a period of 31 days was given. She tolerated radiation therapy well and is currently without symptoms of recurrence of her cancer, and radiation induced changes. She is free of the disease at 12 months of follow-up. Her baby is healthy too without any abnormalities.
Cervical cancer is the most common malignant neoplasm diagnosed during pregnancy and, fortunately, most patients are diagnosed having early stages of the disease [5,10-12]. Treatment for cervical cancer during pregnancy depends on a number of factors, such as the stage of the disease (tumor size), lymph node involvement, gestational age, histological subtype, the patient's desire to continue the pregnancy and the desire for the future fertility spearing [11]. It is important to emphasize that during pregnancy the oncological outcome in patients with cervical cancer are similar in compare with non-pregnant patients, noting that the effect of pregnancy on tumor biology remains until now unclear. Delay in treatment to achieve fetus vitality or improved fetal outcome may be an option for patients with early stage cervical cancer.
Besides surgery, systemic treatment, if compatible with pregnancy, plays an important rolein antenatal oncologic treatment. Neoadjuvant chemotherapy has emerged as an alternative to parallel chemotherapy and radiation therapy, and this may offer patients the opportunity postpones definitive treatment until fetal viability. In this setting based on platinum chemotherapy is the most commonly used systemic therapy in pregnant women. It should be noted that the chemotherapy performed in the second and third trimester may be associated with intrauterine growth retardation, prematurity and low birth weight. In addition, the introduction of chemotherapy within 3 weeks after the intended shipping is not recommended [11]. Our patient, starting from 24 weeks of pregnancy, underwent 3 courses of neoadjuvant cisplatin-containing chemotherapy with an interval of three weeks between courses, with no delay and adverse effects. The last course was completed at 33 weeks of gestation.
There are no studies in the literature on the ideal follow-up option for patients receiving NACT during pregnancy [4]. We performed a follow-up evaluation after completing 3 cycles of NACT, which included pelvic exam, colposcopy and MRI of the pelvic organs. According to these data, almost complete tumor regression was achieved after 3 courses of NACT. A minor residual tumor of the cervix was later confirmed by histological examination.
As far as delivery method is concerned, the literature is mixed. To achieve the ideal approach, several points need to be considered. If possible, delivery should be scheduled by 37 weeks of gestation to avoid prematurity and neonatal complications. Most patients undergo a Caesarean section. The incidence of Cesarean section in pregnant cancer patients is observed in 30%, which is higher compared with a recorded global rate of 21% [11,13]. Vaginal delivery is contraindicated for the most cervical and vulvar cancers because of the potential for implantation of cancer cells in the vaginal tear/episiotomy site [7]. In our patients' case after completed three cycles of neoadjuvant platinum-based chemotherapy at a gestational age of 37 weeks a simultaneous Caesarean section and a radical abdominal hysterectomy were successfully performed.
Cervical cancer diagnosed during pregnancy remains is rare condition [4]. Despite the availability of NCIP recommendations, cancer treatment during pregnancyis still challenging, and decision-making always should be multidisciplinary. If there is a desire to preserve the pregnancy, specialists need to weigh the safety of the mother and child and choose the optimal cancer treatment possible while minimizing the risks to the fetus and mother [4,6,7,11].
The author did not receive a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
O.P.M. was involved in surgical treatment of the patients, analysis of the results and wrote the article.