Endometriosis is a prevalent condition that affects women's health-related quality of life worldwide and deep infiltrating endometriosis represents a subset of these patients who are most severely affected. Due to the complex nature of deep infiltrating endometriosis (DIE) a preoperative suspicion for the condition allows for coordination of a multidisciplinary approach to surgical planning, a key to successful surgical resection. We describe three patient cases to highlight the importance of preoperative planning and the added benefit of imaging with an MRI protocol specific for DIE that includes vaginal and rectal contrast. Additionally, we emphasize the importance of appropriate referral to surgical subspecialists to allow for coordination of care during pre-operative planning to improve outcomes of patients with deep infiltrating endometriosis.
Chronic pelvic pain, Deep infiltrating endometriosis, Appropriate imaging evaluation MRI
DIE: Deep infiltrating endometriosis; EBL: Estimated blood loss; LEEP: Loop electrode excisional procedure; IUD: Intrauterine device; MRI: Magnetic resonance imaging; UTI: Urinary tract infection; TVUS: Transvaginal ultrasound; RES: Rectal endoscopic sonography
Endometriosis is a prevalent condition that affects women's health-related quality of life worldwide. Deep infiltrating endometriosis (DIE) represents a subset of these patients, estimated to affect up to 20% of endometriosis patients [1]. DIE is defined as lesions penetrating at least 5 mm deep to the peritoneal surface and could have genital or extragenital manifestations, including involvement of the uterosacral ligaments, the rectosigmoid colon, the vagina, the bladder, and the small and large bowel [2,3]. In advanced cases, large full-thickness lesions need to be resected from the vagina, bowel, bladder and ureters, resulting in extensive surgeries. For this reason, it can have a profound clinical impact and diagnosis and treatment can be challenging. While patient symptoms and anatomic sites of DIE do correlate, physical exam has limited value in assessing the extent of the disease which frequently requires imaging by ultrasound or MRI, as well as multidisciplinary consultations [4]. The complex nature of DIE mandates taking preoperative suspicion seriously in surgical planning and patient counseling [5,6].
Beyond a thorough pelvic examination, imaging is the modality to fully assess the extent of disease in order to prepare for surgical resection. Sensitivity of ultrasound in detecting DIE with bowel preparation is estimated to be 75-98% [5], however, it is operator dependent and in practices where adequate expertise is not available, detection rates might be lower. The role of MRI is evolving and can be helpful in cases where there is a clinical suspicion of involvement of extragenital organs [7]. Bazot, et al. compared imaging modalities for DIE and concluded that MRI performs similarly to rectal endoscopic sonography (RES) for the diagnosis of intestinal endometriosis, however has higher sensitivity for uterosacral ligament and vaginal endometriosis. The sensitivity of MRI was 84.8% vs. RES 45.6% for detecting uterosacral endometriosis, MRI 77.7% vs. RES 7.4% for detecting vaginal endometriosis, and MRI 88.3% and RES 90% for detecting colorectal endometriosis [8]. MRI is often used for imaging because it is not operator dependent like TVUS, it can pick up peritoneal disease which ultrasound misses, and it helps to determine if visceral involvement, importantly gastrointestinal or genitourinary, is noted. If identified, appropriate referral to specialists can be initiated to assure that surgeons from different specialties are present during surgery, so that all resectable disease is removed to avoid the need for subsequent and repeated surgeries and to improve the patient's quality of life. Given the complex nature of DIE, optimizing outcomes requires extensive preoperative planning and multidisciplinary coordination. In this manuscript, we present the cases of three patients with DIE, to demonstrate the importance of preoperative diagnosis, preoperative planning, and involvement of a multidisciplinary team of surgeons.
The patient is a 35-year-old G2P0 with chronic pelvic pain, failed IVF cycles, and biopsy-proven endometriosis from her prior laparoscopy who presented with worsening pelvic pain and dyschezia. Patient had undergone laparoscopic resection of endometriosis seven years prior, with operative report describing stage 4 endometriosis and significant bowel adhesions. Moreover, she was noted to have biopsy proven endometriosis adjacent to and possibly involving the rectal lesion which was not resected as it was not anticipated pre-operatively and as a result, surgical back up for a colorectal surgeon was not arranged. Her symptoms transiently improved after her first surgery but returned several years later and were not responsive to medical management with combined oral contraceptives. Limited exam in the office due discomfort demonstrated a 14-week globular uterus adherent to the anterior abdominal wall with no other significant findings. Pre-operative MRI with intravenous contrast demonstrated bilateral endometriomas as well as T1-hyperintense implants along the surface of the uterus, cervix, and posterior to the bladder (Figure 1).
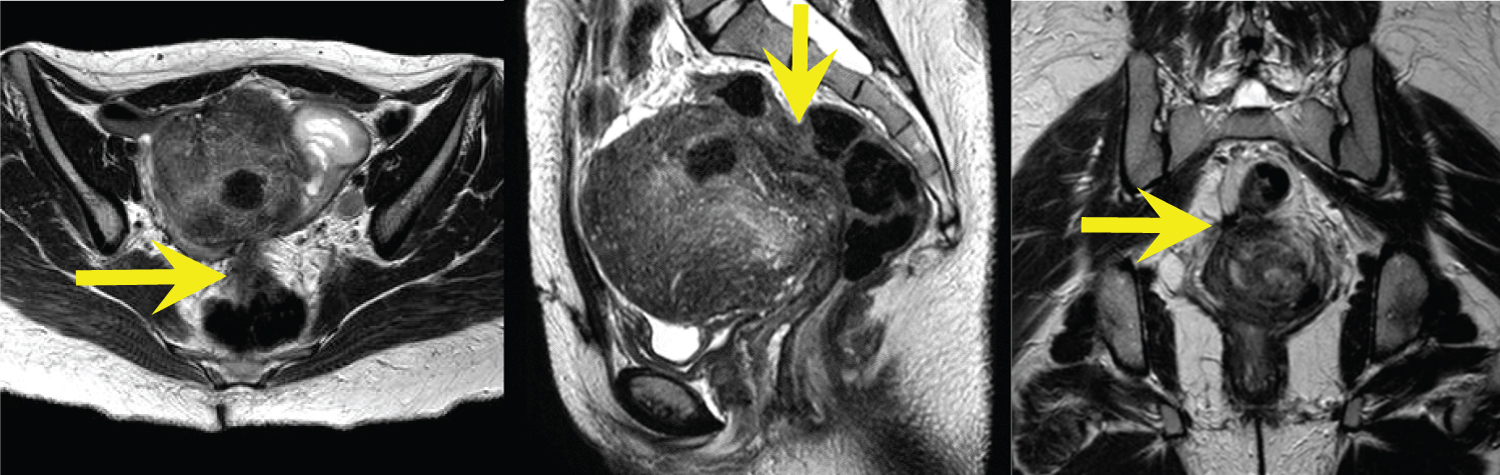 Figure 1(A,B,C): Axial, sagittal, and coronal T2-weighted MR images demonstrate an enlarged uterus with a markedly thickened junctional zone and subcentimeter T2 hyperintense foci throughout the myometrium; findings consistent with adenomyosis. Evaluation of the rectouterine space demonstrates infiltrative T2 hypointense signal abnormality which is inseparable from the anterior rectum, representing DIE with rectal involvement (yellow arrows). View Figure 1
Figure 1(A,B,C): Axial, sagittal, and coronal T2-weighted MR images demonstrate an enlarged uterus with a markedly thickened junctional zone and subcentimeter T2 hyperintense foci throughout the myometrium; findings consistent with adenomyosis. Evaluation of the rectouterine space demonstrates infiltrative T2 hypointense signal abnormality which is inseparable from the anterior rectum, representing DIE with rectal involvement (yellow arrows). View Figure 1
The MRI did not show evidence of intestinal endometriosis, nor did a subsequent colonoscopy when performed pre-operatively. Despite this negative gastrointestinal work up, given the patient's complaints and the report of possible bowel involvement in her last surgery, an outpatient general surgical consultation was obtained, and patient was consented for possible rectosigmoid resection. Finally, pre-operative cystoscopy was performed due to persistent urinary complaints, which was negative for evidence of endometriosis. The patient was extensively counseled, and the plan was made to perform robotic assisted total laparoscopic hysterectomy with bilateral salpingo-oophorectomy and resection of endometriosis.
Exam under anesthesia demonstrated a fixed rectum and uterosacral ligaments. Digital rectal exam was notable for a palpable 2-centimeter lesion on the upper rectum. Inspection of the vagina identified a 2-centimeter nodule in the posterior fornix and a 1 cm anterior vaginal wall nodule halfway between the introitus and apex. Intraoperatively, presence of bilateral endometriomas was confirmed (10 centimeters on the left and 3 centimeters on the right). A "frozen pelvis" was noted with dense pelvic adhesions and an obliterated posterior cul-de-sac. Finally, nodules typical for DIE were seen on the rectosigmoid, correlating with exam, as well as on the terminal ileum, and the appendix.
Extensive dissection was performed as follows: the retroperitoneum was entered bilaterally via lateral approach to the sidewalls, pararectal spaces were developed, and bilateral ureterolysis performed. Lysis of adhesions of the uterus and adnexa was performed to mobilize the uterus and adnexa and to separate it from sidewall and vital structures. Extensive fibrosis was noted, making it difficult to identify individual peritoneal implants. At the conclusion of the hysterectomy, an upper partial vaginectomy was performed to remove vaginal endometriosis lesions seen on exam. Next, appendectomy as well as a discoid resection of the rectosigmoid lesion, located at approximately 11 cm from the anal verge, were performed by the general surgeon. An anterior and lateral mobilization of the rectum was performed and superficial lesions of the mesorectum were excised. A few small fibrotic areas were noted on the colon and terminal ileum and biopsies were performed. A nerve-sparing approach was taken throughout the above dissection with identification of the hypogastric plexus.
Total surgical time for the surgery was 461 minutes. Estimated blood loss (EBL) was 500 milliliters. Post-operative course was complicated by acute blood loss anemia for which the patient received 3 units of packed red blood cells with subsequent stabilization of hematocrit. Post-operative course otherwise uneventful and after the patient was discharged home on postoperative day 2. Patient did well clinically and was pain free at her 6-week post-operative visit.
Pathology review of specimens confirmed the presence of endometriosis within the resected portions of the vagina and the rectosigmoid. The biopsy of the small bowel, also was positive for endometriosis. The appendix contained subserosal fibrosis with hemosiderin-laden macrophages but no overt endometrial glands and stroma was reported. In light of the bowel involvement that was not described on imaging preoperatively, the MRI images was re-reviewed with radiology, and rectal involvement was retrospectively appreciated (Figure 2).
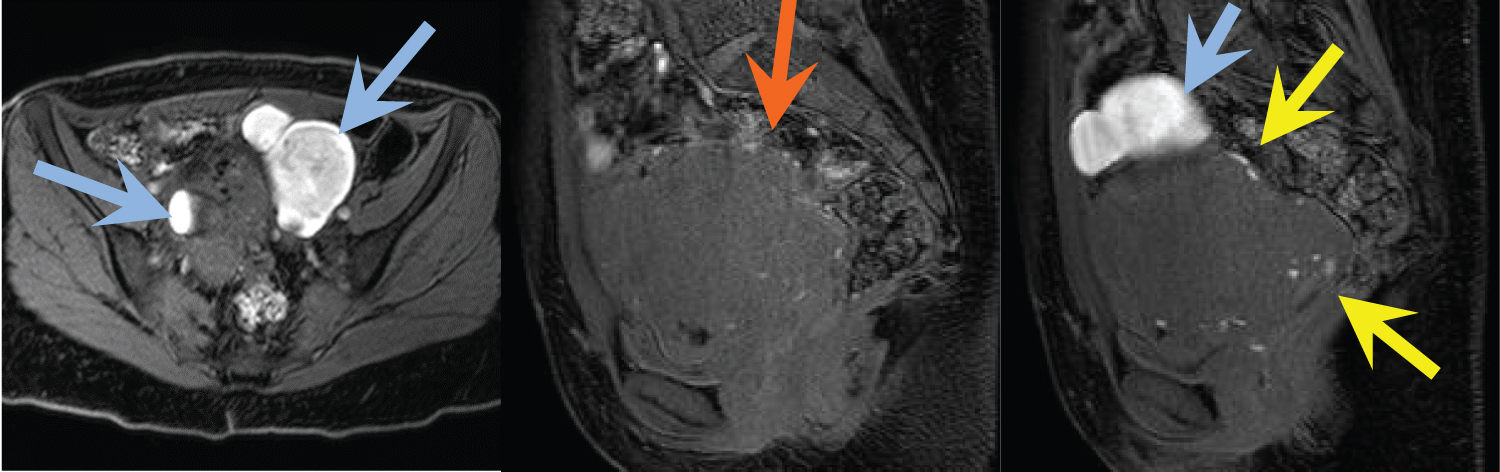 Figure 2(A,B,C): Axial and sagittal fat-suppressed, T1-weighted MR images demonstrate bilateral T1 hyperintense ovarian masses consistent with endometriomas (blue arrows). The sagittal images show numerous sub-centimeter T1 hyperintense foci along the periphery of the uterus, representing endometrial implants (yellow arrows). Some of these foci are noted along the serosa of the rectum in the location of DIE identified on the T2 images (orange arrow). View Figure 2
Figure 2(A,B,C): Axial and sagittal fat-suppressed, T1-weighted MR images demonstrate bilateral T1 hyperintense ovarian masses consistent with endometriomas (blue arrows). The sagittal images show numerous sub-centimeter T1 hyperintense foci along the periphery of the uterus, representing endometrial implants (yellow arrows). Some of these foci are noted along the serosa of the rectum in the location of DIE identified on the T2 images (orange arrow). View Figure 2
41-year-old G4P0040 with a 15-year history of chronic pelvic pain with symptoms of dysmenorrhea, dyschezia, constipation, dysuria, and abnormal uterine bleeding failed previous management with hormonal suppression. Her history was significant for irritable bowel syndrome, depression, alcoholism, and a prior laparoscopic excision of endometriosis and appendectomy. Given her bladder and bowel symptoms, pre-operatively the patient was seen by both urology and general surgery. Colonoscopy noted a 3-centimeter non-obstructing lesion protruding into but not all the way through mucosa with negative mucosal biopsies. Pre-operative cystoscopy was negative.
On exam in the office, she was noted to have a 10-week sized uterus with mobility limited by posterior cul-de-sac lesion with a 2-centimeter posterior fornix lesion tethered to a rectal lesion. The MRI, which this time was performed with intravenous, oral, rectal, and vaginal contrast, reported deep pelvic endometriosis with mass like T2-hypointense process in the rectovaginal septum, with gross invasion into the anterior rectal wall. The endometrial mass appeared to invade full thickness of the rectal wall, extending into the rectal lumen (Figure 3 and Figure 4). MRI also demonstrated adenomyosis and thickening of the right fallopian tube and right proximal round ligament.
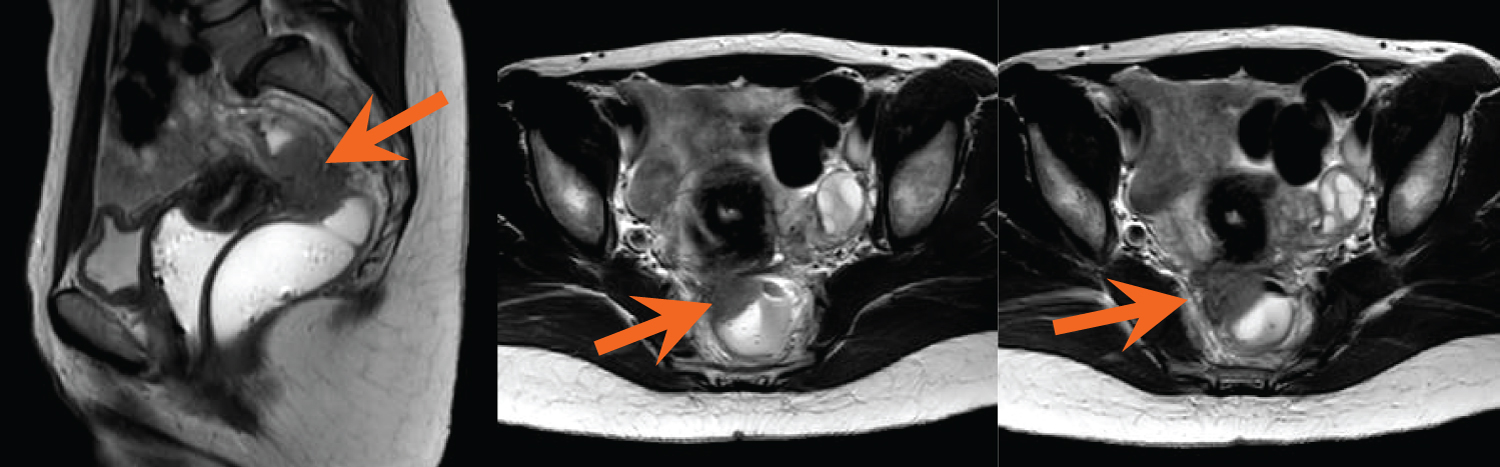 Figure 3(A,B,C): Axial and sagittal T2-weighted MR images show a large mass of intermediate signal intensity involving the upper rectovaginal septum and invading the rectum (arrows). Hypointense spiculation within the surrounding fat corresponds to fibrotic reaction. Of note, the rectum and vagina are distended with gel per exam protocol. View Figure 3
Figure 3(A,B,C): Axial and sagittal T2-weighted MR images show a large mass of intermediate signal intensity involving the upper rectovaginal septum and invading the rectum (arrows). Hypointense spiculation within the surrounding fat corresponds to fibrotic reaction. Of note, the rectum and vagina are distended with gel per exam protocol. View Figure 3
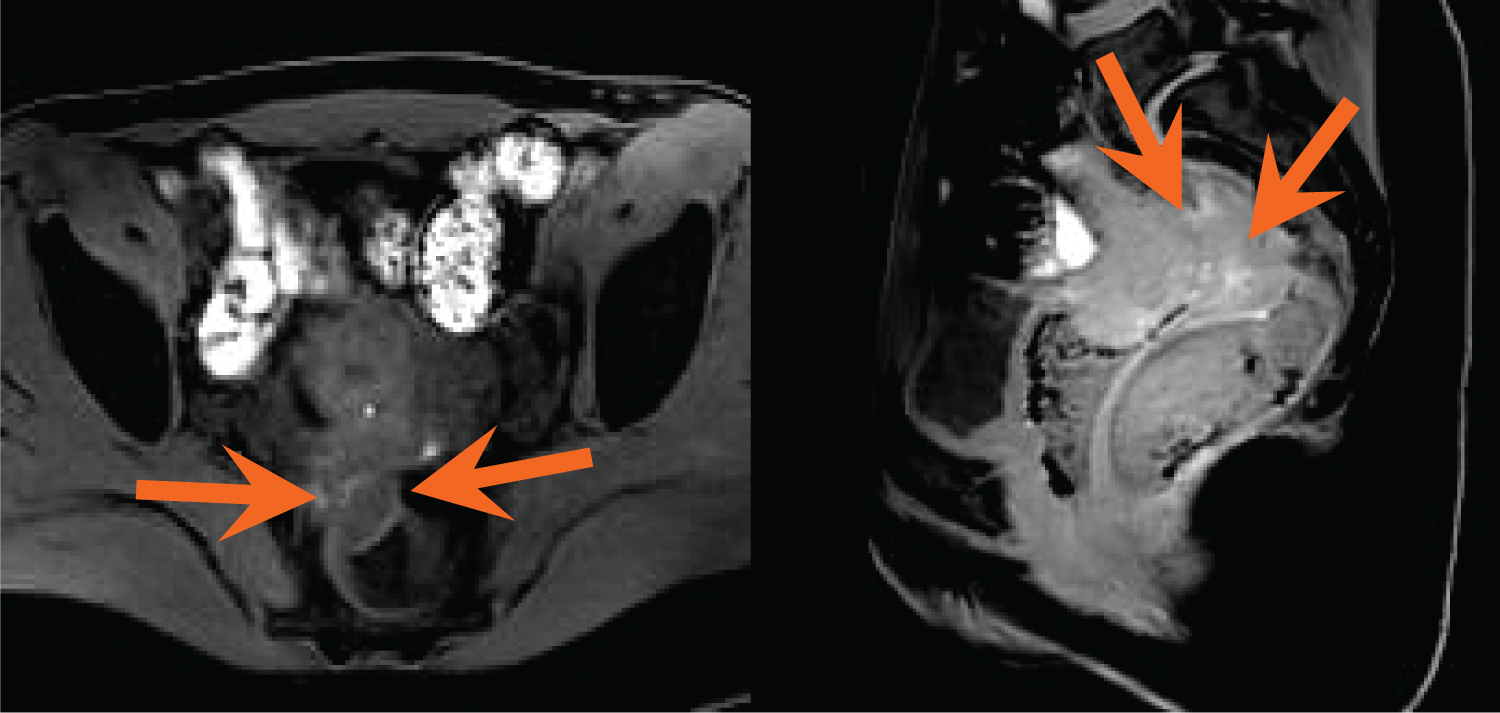 Figure 4(A,B): Axial and sagittal fat-suppressed, T1-weighted MR images demonstrate scattered T1 hyperintense foci within the pelvis, including within the rectal mass (orange arrows); findings are consistent with DIE. View Figure 4
Figure 4(A,B): Axial and sagittal fat-suppressed, T1-weighted MR images demonstrate scattered T1 hyperintense foci within the pelvis, including within the rectal mass (orange arrows); findings are consistent with DIE. View Figure 4
Pre-operatively, both general surgery and gynecology surgeons had extensive discussions with the patient regarding etiology, treatment options and surgical options. She did not desire childbearing but desired ovarian conservation to avoid premature menopause. She then underwent a robotically-assisted total laparoscopic hysterectomy, bilateral salpingectomy, cystoscopy, bilateral ureterolysis, lysis of adhesions, segmental rectosigmoidectomy and anastomosis, partial vaginectomy, and flexible sigmoidoscopy. EBL for the case was 500 cc. Findings at the time of surgery were notable for a 2-centimeter mid-upper vaginal posterior fornix nodule with blue-brown hue consistent with transmural vaginal endometriosis nodule, 12-week sized globular uterus consistent with adenomyosis, normal adnexa bilaterally, DIE endometriosis lesions limited to rectosigmoid (5 × 3 centimeters), upper vagina (3 × 3 centimeters), bilateral uterosacral ligaments, 10 centimeters in aggregate, not noted in other areas. Dense adhesions and obliterated posterior cul-de-sac were noted in the pelvis. At conclusion of the procedure, all grossly visible endometriosis had been completely resected. Pathology was consistent with endometriosis in all specimens observed during surgery and the uterus was consistent with adenomyosis and leiomyomata.
Post-operative course was complicated by post-operative urinary retention, presumably secondary to disruption of the parasympathetic plexus during the deep endometriosis resection for which she required intermittent catheterization with timed voids.
Case 3 is a 33-year-old G1P1001 with past history significant for a cesarean section, LEEP, history of chlamydia, and CopperT IUD in place. She had presented as a referral from urology for chronic pelvic pain and an endometriosis bladder lesion that was seen on outpatient cystoscopy. The plan was for a combined procedure. The patient complained of dysmenorrhea and chronic pelvic pain with urinary symptoms; bladder irritation, dysuria, urinary frequency, and frequent urgent visits for UTI symptoms with negative urine cultures. Her chronic pelvic pain was cyclical. Exam in the office was unremarkable. Pre-operative office cystoscopy demonstrated no stones or diverticula but identified a blue hue endometrial nodule covered with bladder mucosa pushing into the bladder posteriorly in the midline. Bilateral ureteral orifices visualized with clear efflux of urine. MRI urogram was done in order to delineate collecting system due to concern with potential ureteral involvement and/or obstruction, which demonstrated a small bilateral T1 markedly hyperintense ovarian cystic structures suspicious for endometriomas and small filling of the bladder posteriorly with contrast without gross visible defect (Figure 5 and Figure 6). Based on the patient's clinical picture a combined procedure was planned with urology. She wanted to preserve her fertility for future childbearing. On exam under anesthesia, a small mobile retroverted uterus with bilateral adnexal fullness was noted with no rectovaginal nodules. Copper T IUD was removed and replaced by Levonorgestrel IUD to reduce recurrence of endometriosis. After cystoscopy with bilateral ureteral stent insertion, robotically-assisted laparoscopy was performed with lysis of adhesions, resection of endometriosis and left ovarian cystectomy, for a 1.5-centimeter ovarian endometrioma. The urology team resected a transmural 3 × 3-centimeter bladder DIE lesion with adequate margins and no vaginal involvement. The bladder defect was closed and confirmed to be water-tight, right ureteral stent was removed but the left ureteral stent was left in place due to its proximity to the suture line. Final pelvic and abdominal survey confirmed little to no residual disease with adequate hemostasis and an EBL 150 cc (Figure 7). Post-operative course was complicated by a urinary tract infection which responded to antibiotics. Pathology confirmed histologic evidence of endometriosis in all excised tissue. Two weeks post-operative, the patient had normal CT cystogram and office cystoscopy and the left ureteral stent was removed without difficulty.
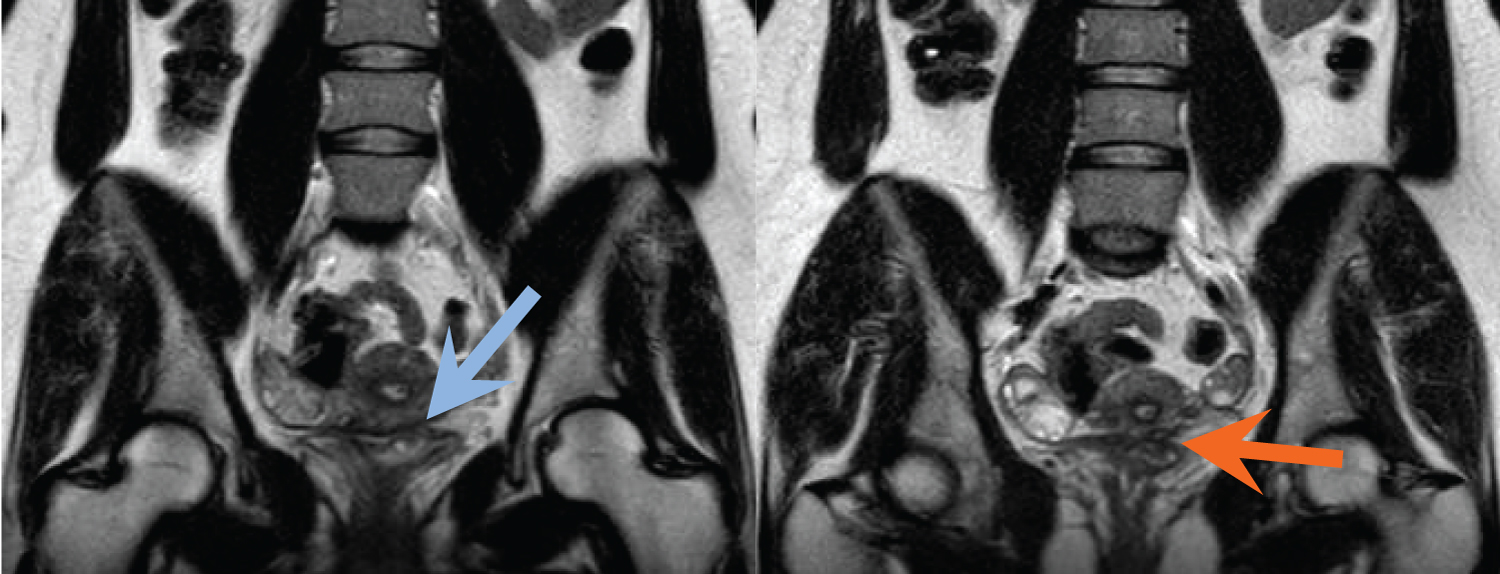 Figure 5(A,B): Coronal T2-weighted images reveal focal soft tissue thickening of the posterior bladder dome, just to the left of midline, with a T2 hyperintense cystic focus (blue arrow) and adjacent infiltrative T2 hypointense signal abnormality within the vesico uterine space (orange arrow). View Figure 5
Figure 5(A,B): Coronal T2-weighted images reveal focal soft tissue thickening of the posterior bladder dome, just to the left of midline, with a T2 hyperintense cystic focus (blue arrow) and adjacent infiltrative T2 hypointense signal abnormality within the vesico uterine space (orange arrow). View Figure 5
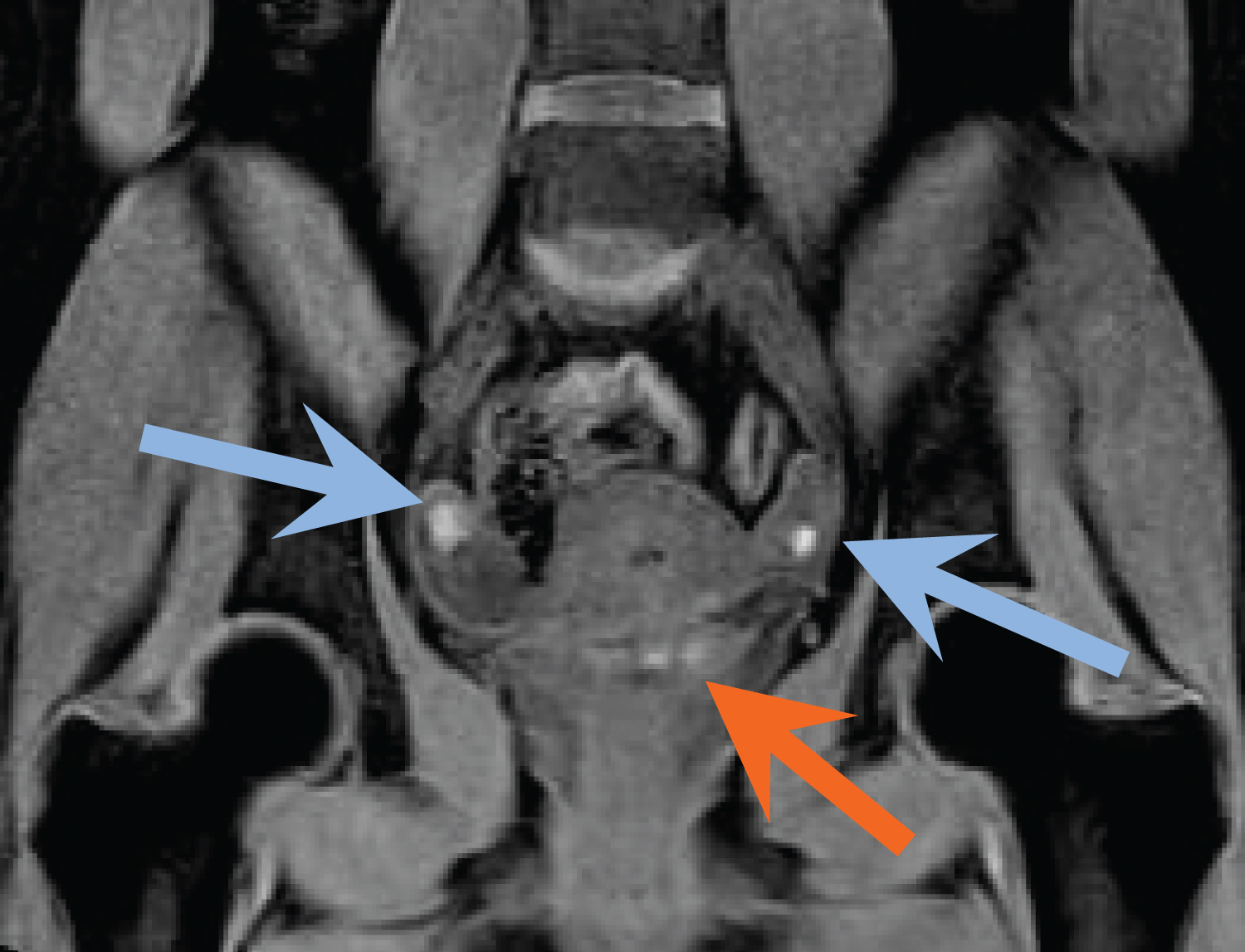 Figure 6: Coronal fat-suppressed, T1-weighted MR image demonstrates small bilateral T1 hyperintense ovarian masses consistent with endometriomas (blue arrows). T1 hyperintense foci are also identified within the posterior bladder dome (orange arrow). Findings represent DIE involving the urinary bladder. View Figure 6
Figure 6: Coronal fat-suppressed, T1-weighted MR image demonstrates small bilateral T1 hyperintense ovarian masses consistent with endometriomas (blue arrows). T1 hyperintense foci are also identified within the posterior bladder dome (orange arrow). Findings represent DIE involving the urinary bladder. View Figure 6
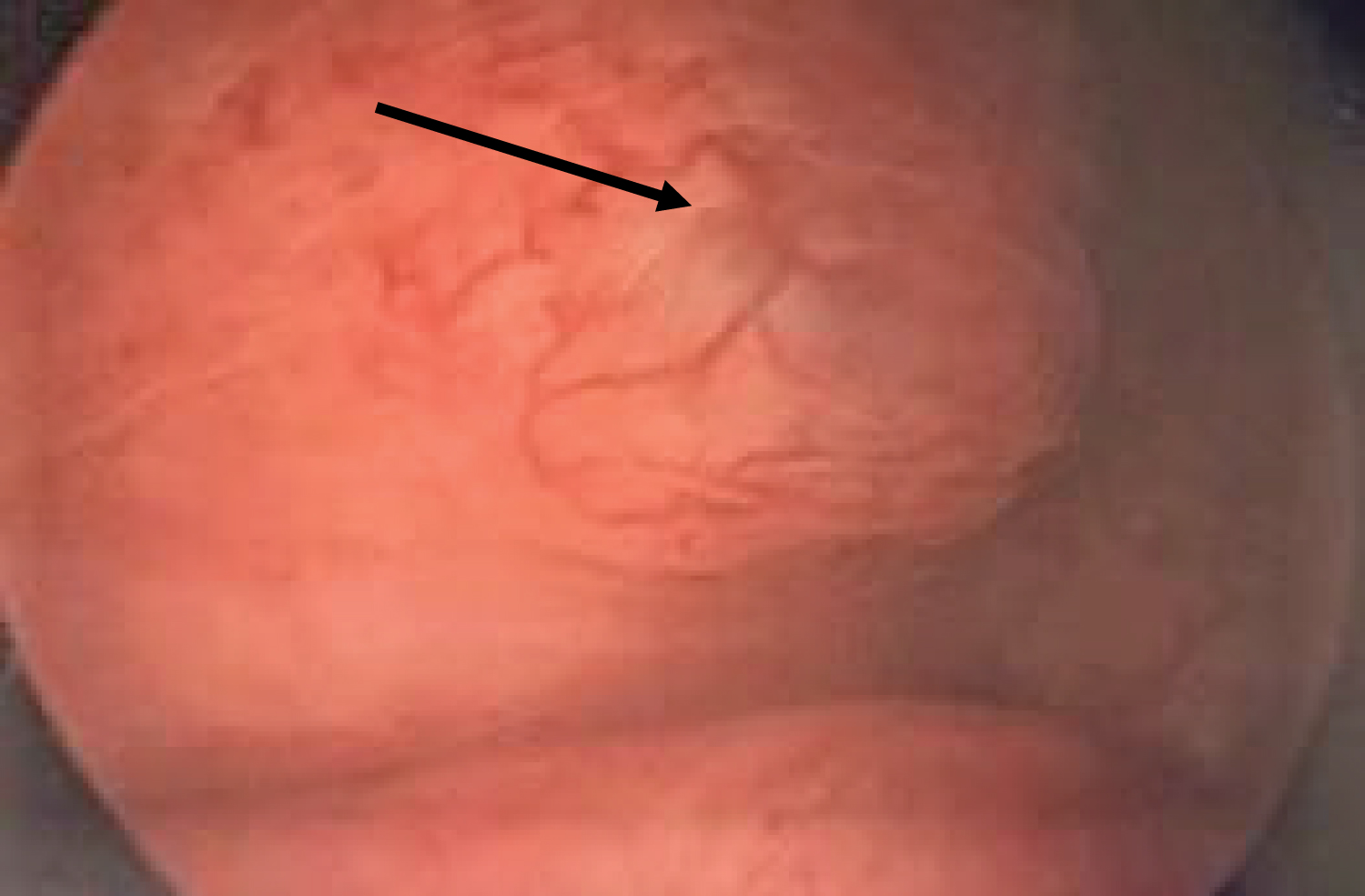 Figure 7: Finding of bladder endometriosis on cystoscopy. View Figure 7
Figure 7: Finding of bladder endometriosis on cystoscopy. View Figure 7
These three cases highlight the importance of extensive preoperative planning by a multidisciplinary surgical team to achieve optimal surgical resection of DIE. Each case demonstrates the value of appropriate preoperative imaging, subspecialty consultation, coordination of care, and an individualized comprehensive work up including colonoscopy or cystoscopy.
While transvaginal ultrasound, cystoscopy, and rectosigmoidoscopy, have been studied and used for the preoperative evaluation of symptomatic endometriosis, MRI may provide an added benefit of mapping deep lesions with greater accuracy than other modalities [9]. Bazot, et al. demonstrated that MRI had a sensitivity of 90.3% and NPV of 89% for DIE which appeared as a hyperintense foci and/or hypointense areas on T1- and T2-weighted MR images, respectively. Similarly, Hottat, et al. reported a sensitivity of 96.3% and NPV of 93.3% [4,9,10]. The use of T1 and T2 weight sequences in mapping lesions has become an integral step in surgical planning for DIE as was demonstrated in the above cases. More importantly, the cases above support the utility of adding vaginal and rectal contrast to the protocol for MRI when evaluating for DIE. Chasong, et al. demonstrated that opacification of the vagina and rectum with ultrasound gel improved the sensitivity of MRI for the detection of DIE and allowed for improved visualization and delineation of the vagina and rectovaginal septum, allowing for better delineation of the pelvic organs [11]. Endometriotic lesions have an MRI signal intensity similar to their surrounding fibromuscular structures, since they are fibromuscular structures. Vaginal and rectal distension and opacification with ultrasound gel can help to delineate the cervix, vaginal fornices, vaginal wall, rectum, and rectosigmoid colon junction [12]. Endelarae, et al. was also in favor of using vaginal and rectal distension to detect and localize, with better accuracy, endometriotic lesions, and to identify conditions either developing inside the lumen of cavities or coming from the outside [13]. For our case 1, DIE discovered intra-op was not seen pre-operatively by MRI most likely because of the lack of vaginal and rectal contrast when obtaining this MRI. After this case the vaginal and rectal contrast was added to the protocol. In case 2, where vaginal and rectal contrast was used for the MRI protocol, DIE was seen involving the rectovaginal septum, with gross invasion into the anterior rectal wall. This allowed for preoperative planning with general surgery and plan for segmental rectosigmoidectomy and anastomosis. These cases prompted the development of a collaborative relationship with radiology and the establishment of formalized reporting system and endometriosis-specific MRI protocol when evaluating for DIE, allowing clinicians to order correct studies and for radiologists to report their findings in a standardized fashion. The inclusion of a radiologist that specializes in MRI is crucial to the multidisciplinary team approach.
When approaching bowel endometriosis, Abrao, et al. describes several considerations critical to surgical planning: The number of DIE lesions, multifocality, lesion size (with lesions over 3 centimeters typically requiring a segmental resection while smaller lesions are often amenable to discoid resections), the extent of bowel surface involvement, and lesion depth [14]. In case 2, the general surgery team identified a greater then 5 centimeters lesion on the upper and medial rectum. Given its size, the lesion was not amenable to shaving or a discoid resection, but rather demanded a segmental resection. In comparison, in case 1 the rectosigmoid lesion was smaller and therefore amenable to a discoid resection. A collaborative relationship with joint meetings to review patient cases was created with general surgery, radiology, and minimally invasive gynecology to improve patient care. As a result, recognition in the community was established and increased referrals were seen, giving us reassurance that patients have better access to comprehensive surgical teams and are avoiding incomplete surgical debulking's.
These three cases highlight the importance of thorough preoperative planning and the added benefit of MRI in preoperative planning with a protocol that includes vaginal and rectal contrast. In addition, we emphasize the importance of referral to subspeciality surgeons to allow for coordination of a multidisciplinary approach to surgical planning to improve treatment and outcomes of patients with deep infiltrating endometriosis. Finally, patients with deep infiltrating endometriosis would benefit from a center with multispecialty providers that can work in conjunction to coordinate patient care and surgical planning.
The authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal's requirements for authorship.
This work was completed without any external source of funding.
We acknowledge that all the authors have contributed to this paper.