Complete hydatidiform mole with co-existing fetus (CHMCF) is a rare obstetrical finding. These pregnancies pose a unique set of health concerns including hemorrhage, thyrotoxicosis, preeclampsia, and gestational trophoblastic neoplasia (GTN). In this report, we present a 34-year-old G5P1 presented at 6 weeks and 5 days with vaginal bleeding. Further follow-up revealed a complete hydatidiform mole with co-existing fetus in a patient who desired continuation of pregnancy. There is a lack of data and evidence surrounding appropriate and safe management with CHMCF. In the correct patient, these pregnancies can be managed safely and carried to term with close postpartum surveillance for GTN.
Complete hydatidiform mole with co-existing fetus, Gestational trophoblastic neoplasia
Complete hydatidiform mole with co-existing fetus (CHMCF) is a rare obstetrical finding, only occurring in 1 per 20,000 to 100,000 pregnancies [1-5]. Historically, it was not recommended that these pregnancies be carried to viability given the high risk of maternal morbidity. Though rare, cases of CHMCF force patients and providers to make difficult risk-benefit decisions. As a result, management guidelines are lacking and continue to evolve.
Complications associated with CHMCF include spontaneous abortion, hyperthyroidism, preeclampsia, antepartum hemorrhage, fetal demise, and gestational trophoblastic neoplasia (GTN) [4].
Perhaps the most worrisome outcome related to CHMCF is the risk of developing life-threatening GTN, either during the antepartum period or remote from delivery. In previously reported cases of CHMCF, there was no increased risk of GTN in patients who terminated a CHMCF versus those who elected expectant management [1].
Here, we present a case outlining management, delivery, and surveillance of a CHMCF in a woman who was offered and chose expectant management of the pregnancy.
A 34-year-old G5P1 at 6 weeks and 5 days presented to her local emergency department with moderate vaginal bleeding along with nausea, pelvic pain, and fatigue. Ultrasound at the time of presentation demonstrated a normal intrauterine pregnancy with a mass consistent with a subchorionic hematoma. She then initiated routine prenatal care. Maternal-Fetal Medicine performed an ultrasound at 11 weeks and 2 days gestation demonstrating a single intrauterine pregnancy with cardiac activity as well as a cystic placenta measuring 7.6 × 6.2 × 4.5 cm, suggestive of molar pregnancy versus subchorionic hematoma related to resolving twin gestation. Follow-up ultrasound was performed at 12 weeks and 2 days, which demonstrated a normal intrauterine pregnancy with a fundal placenta normal in appearance. Additionally, an anterior mass was noted that was increasingly hydropic in appearance from previous scans with no associated fetus. Quantitative b-hCG (b-hCG) at this gestational age was 181,000 mIU/mL. Given the sonographic and serologic findings consistent with molar pregnancy, the patient was recommended termination of pregnancy due to the high risk of disease progression and hypertensive disorders of pregnancy. The patient and her significant other desired to continue the pregnancy, and she was referred to a tertiary care center at 13 weeks of gestation for consultation with Gynecology Oncology. During this consultation, the patient was counseled about the malignant potential of GTN and inability to administer recommended chemotherapeutic agents due to teratogenicity. Additionally, she was counseled that by electing to continue with pregnancy, she could develop metastatic disease, which would become less curable. Her provider discussed with her the option to proceed with imaging of the chest, abdomen, pelvis, and head to assess for spread of disease. Given that the patient desired to continue the pregnancy, the decision was made to manage expectantly with the plan to image further during pregnancy only based on symptoms.
At 16 weeks and 5 days gestation, the patient's care was transferred to a tertiary care center for further management. The patient was counseled by Maternal-Fetal Medicine. She was offered and elected expectant management with close monitoring. A detailed obstetric anatomy ultrasound was also performed at this time which demonstrated a non-anomalous fetus with normal anterior placentation, and a placental mass concerning for gestational mole seen on the anterior left uterine wall abutting the normal-appearing placenta (Figure 1). She was recommended and underwent genetic amniocentesis, which later returned as normal male fetus (46XY). The patient was followed in the outpatient setting every 1-2 weeks with serial fetal growth scans and twice weekly antenatal testing. The patient was instructed to monitor blood pressures at home. Clinical parameters remained stable and reassuring throughout pregnancy and the patient was recommended planned delivery at 37 weeks. She underwent uncomplicated scheduled primary cesarean delivery for breech presentation at 37 weeks 0 days, resulting in delivery of a viable neonate weighing 2730 grams with APGARs of 7 and 9. Umbilical artery and venous pH were 7.25 and 7.32, respectively. Following delivery of the placenta, a hydropic mass consistent with complete mole was delivered (Figure 2) without complications. Histologic examination of the mass confirmed the pathologic diagnosis. Diagnosis was further confirmed by negative p57 staining. Tissue chromosomal analysis demonstrated 46XY karyotype.
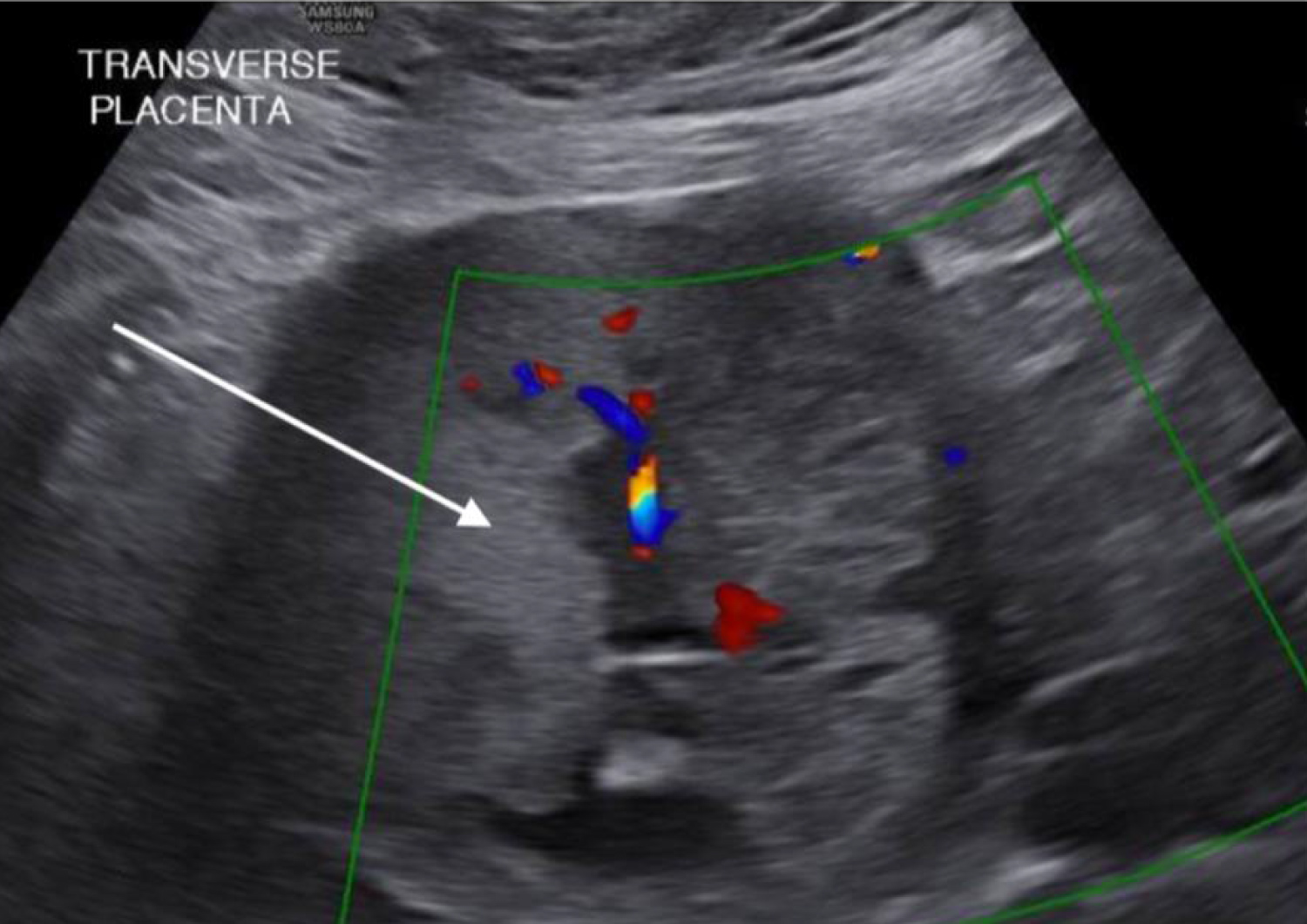 Figure 1: Ultrasound findings. Ultrasonographic findings consistent with non-anomalous male fetus and co-existing complete hydatidiform mole abutting normal appearing placenta. Arrows depicting complete hydatidiform mole.
View Figure 1
Figure 1: Ultrasound findings. Ultrasonographic findings consistent with non-anomalous male fetus and co-existing complete hydatidiform mole abutting normal appearing placenta. Arrows depicting complete hydatidiform mole.
View Figure 1
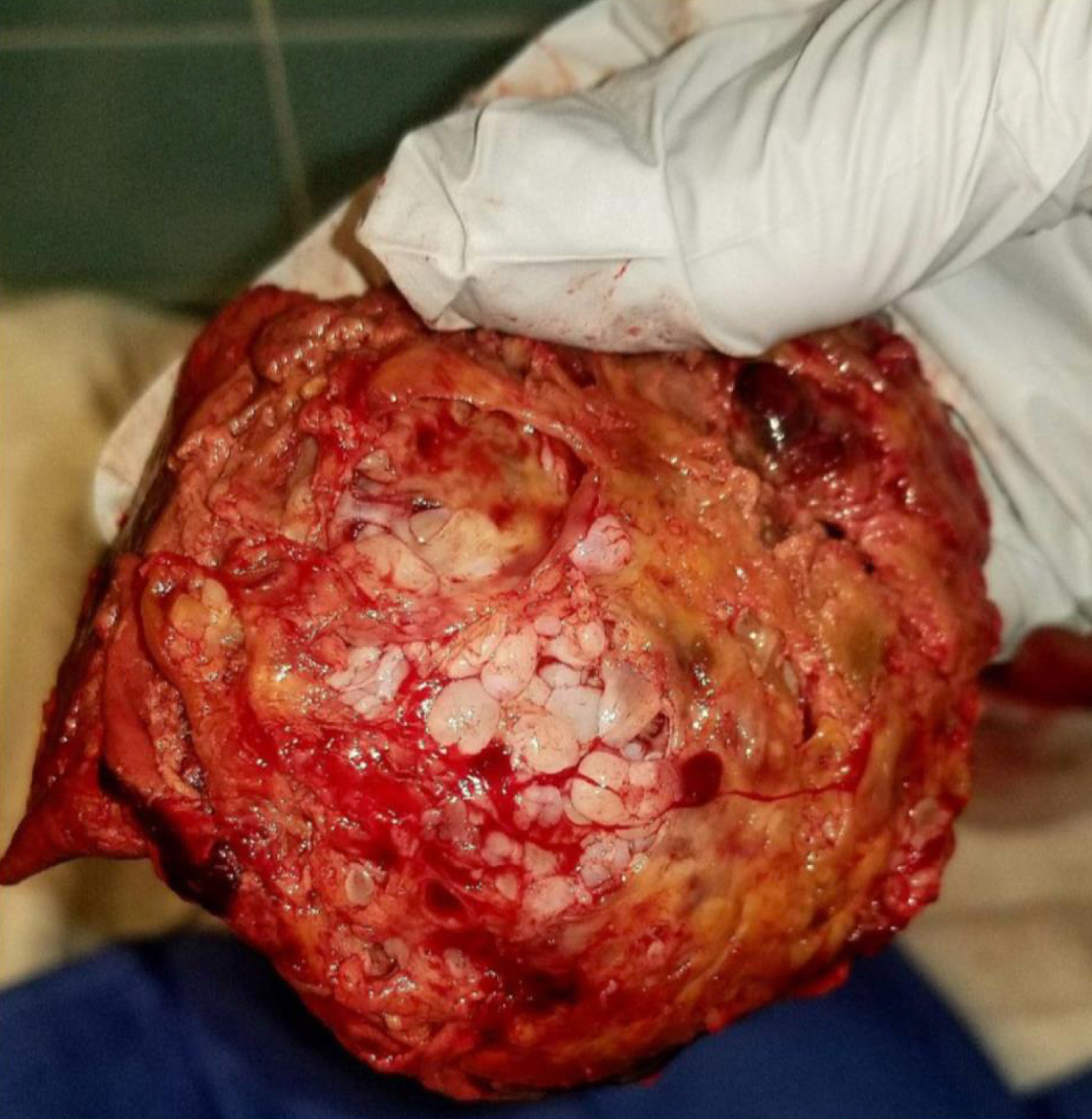 Figure 2: Gross appearance. Gross appearance of complete hydatidiform mole at the time of cesarean delivery.
View Figure 2
Figure 2: Gross appearance. Gross appearance of complete hydatidiform mole at the time of cesarean delivery.
View Figure 2
The importance of postpartum surveillance and follow-up was discussed with the patient. Per the American College of Obstetricians and Gynecologists recommendations on post-evacuation management of molar pregnancy, the patient was recommended weekly assessment of quantitative serum b-hCG levels until negative for three consecutive weeks. Serum b-hCG levels would then be assessed monthly for six consecutive months. If during the surveillance period, serum levels remained negative and she remained asymptomatic, she would then be recommended routine gynecologic follow-up [6]. The patient was compliant with weekly follow-up until quantitative b-hCG level reached zero, 44 days following delivery. Unfortunately, she did not present for her monthly visits after that point despite continued telephone counseling. Eventually, she was lost to follow-up and was sent a certified letter outlining her risks of non-compliance.
Pregnancies complicated by CHMCF are rare, and as a result, there is a paucity of literature to help guide management. Furthermore, reported outcomes in the setting of CHMCF vary significantly. In one case report of CHMCF published by Vandenhove, et al., the patient initially elected expectant management but ultimately underwent termination via dilation and curettage at 18 weeks due to severe vaginal bleeding. She then developed gestational trophoblastic neoplasia and required treatment with methotrexate [7]. In another documented case report of CHMCF published by Buke, et al., a desired pregnancy complicated by CHMCF was described wherein the patient presented at 17 weeks and 4 days with vaginal bleeding. Clinical evaluation revealed CHMCF complicated by complete placenta previa. The patient strongly desired expectant management and she was discharged home with precautions. She then presented at 32 weeks gestation with vaginal bleeding, received a course of betamethasone, and underwent cesarean delivery of a live neonate. She was discharged to home post-operatively and underwent recommended surveillance without any further complications [8].
GTN is a known potential complication of CHMCF that often prompts providers to recommend termination, with some literature describing the incidence of GTN after CHMCF to be as high as 37% [4]. Despite this feared complication, there is evidence that termination of CHMFC does not decrease the risk of GTN. Sebire, et al., reported 19.5% of 77 pregnant women with CHMCF developed GTN with no difference in outcome between pregnancies undergoing termination in the first trimester versus those that continued through the second trimester [3]. It is important to note, however, that diagnostic criteria and treatment of GTN varies in the United Kingdom as compared to the United States, with a more conservative approach in the United Kingdom. Data regarding less conservative management and outcome of cases of complete hydatidiform mole with co-existing fetus remains lacking.
The risk of malignant change in a pregnancy complicated by a complete mole is approximately 15-20% [4]. Additionally, twin pregnancies with a fetus and a mole are at a higher risk of post-molar gestational trophoblastic neoplasia as compared to singleton hydatidiform moles [9]. Many classification systems have been proposed to prognosticate patients with gestational neoplasia, including the World Health Organization (WHO) prognostic index score, the Clinical Classification System developed at the NIH, and the FIGO staging system. The Clinical Classification system is most frequently used in the United States. This system segregates patients based on metastatic status, as almost all patients with no known metastatic disease can be cured with single-agent chemotherapy. The cure rate for non-metastatic disease is approximately 100%. Furthermore, those with metastatic disease can be further classified as having low-risk or high-risk metastatic gestational trophoblastic disease. Those with one or more risk factors are classified as high-risk. These patients are recommended multi agent chemotherapy due to increased risk of failure and death if single-agent chemotherapy is used. High risk factors include a long duration (> 4 months) since last pregnancy, pre-therapy hCG levels of > 40,000 mIU/mL, brain or liver metastases, antecedent term pregnancy, or prior chemotherapy [6]. Almost all patients with low-risk disease are curable. Those with high-risk disease have a survival rate as high as 84% [9].
With this report, we present a case of CHMCF delivered at term. In this case, we recommended baseline chest imaging at the time of initial consultation. Monitoring of b-Hcg and imaging surveillance during the pregnancy was deferred given the patient's strong desire for non-intervention with regard to molar pregnancy. Though our patient was lost to follow-up, following serum quantitative levels for six months after achieving a level of zero in the postpartum period is recommended.
Patients affected by CHMCF must be counseled about the associated risks related to the pregnancy as well as long-term oncologic risks and the lack of data surrounding management. Our experience suggests that, in very specific situations, it is reasonable to offer expectant management of CHMCF with delivery at term. More data is needed regarding the incidence of obstetric morbidity, disease progression, and treatment efficacy compared to early termination in the setting of CHMCF.