Lead is a toxic heavy metal and its relation to hypertension is well-known. Conventional medications which are used to treat lead toxicity are teratogenic and contra-indicated in pregnancy.
To measure blood lead levels (BLLs) in preeclampsia patients and to assess the effect of N-acetyl cysteine (NAC) supplementation on these patients.
Blood lead levels were measured in 115 pregnant women suffering from preeclampsia (PE) and were compared with its levels in 25 full-term healthy pregnant women. It was measured using atomic absorption spectrophotometry (AAS). Then, NAC was supplemented to PE cases with their conventional medications. BLLs were re-tested again after four and six weeks of giving the supplement and were compared with their levels in PE cases who received the conventional medications only without NAC supplementation.
BLL in the PE women was 37.68 ± 9.17 µg/dl, with a highly significant difference (p < 0.001) with the comparison group of healthy pregnant women (14.5 ± 3.18 μg/dl). There was found significant decreases in the BLLs, the blood pressure and the degree of proteinuria in PE cases who received NAC supplement for one month or more (P = 0.00, 0.03, 0.00 respectively). There was found a highly significant positive correlation between BLLs and elevated blood pressure and proteinuria in the cases (correlation coefficient = 0.01).
N-acetyl cysteine is a potent antioxidant that proved to be promising in decreasing the severity of lead toxicity and of elevated blood lead levels in pregnancy. N-acetyl cysteine supplementation is found to be associated with a decrease in the elevated blood pressure and proteinuria which are two of the hallmarks of preeclampsia.
Preeclampsia, Blood lead levels, N-acetylcysteine, Proteiuria, Elevated blood pressure
Lead toxicity is still a prevalent public health problem in both developed and developing countries. While, the developed countries have largely succeeded to decrease the severity of this problem, developing countries still suffer from the burden of this health hazard and many sources of lead exposure still exist in these countries [1-3].
Lead is toxic to many organs and systems in the body. Lead is well-linked to hypertension. This may be mediated by renal, vascular, hormonal or oxidative stress pathways. The exact mechanism by which lead causes hypertension is still not well-known, but there stands a truth that 'Lead causes hypertension' [4]. Hypertension endangers organ perfusion. This is particularly risky in pregnancy for both the mother and the fetus. One of the hypertensive disorders of pregnancy and of the commonest pregnancy disorders is preeclampsia (PE) [5].
Preeclampsia is still a disease of theories. Oxidative stress is one of the theories proposed to explain the incidence of PE. NAC is a pharmaceutical supplement that has powerful antioxidant properties; being a precursor of the glutathione peroxidase enzyme. NAC has many benefits that are still investigational, for example, its observed beneficial role in polycystic ovarian syndrome (PCOD) [6].
Fiore and Capasso, stated that the antioxidant effects of NAC in preventing and treating oxidative stress state in preeclampsia are not widely investigated, and their optimum dosing and timing for pregnant women need further research [7].
This study is a prospective randomized controlled clinical trial. Subjects contributing in this study were divided into:
A Control Group: Twenty-five (25) full-term healthy pregnant women.
A Test Group: One hundred fifteen (115) pregnant women suffering from PE. The pregnant women were recruited from Mansoura University Hospital, Obstetrics/Gynecology Department. An Approval of the local ethical committee of the university and patient consents were obtained after explaining the study objectives to them.
Preeclampsia cases were selected among pregnant women suffering from hypertension and proteinuria after 20 weeks' gestation, and who are decided by the obstetrician to be maintained on medical treatment for more than one month.
One hundred patients had completed the study. They were divided into two groups:
Group I (n = 50): Pregnant women with preeclampsia who received N-acetyl cysteine supplementation in a dose of 400 mg/day [8].
Group II (n = 50): Pregnant women with preeclampsia who did not receive N-acetyl cysteine supplementation (a positive control group).
The supplementary therapy of NAC was given with the conventional medications of preeclampsia which included [9]:
• Antihypertensive medications: Aldomet 250 mg tablets (α-methyl dopa).
• Epilat retard capsules (Calcium-channel blocker) was given if hypertension failed to be controlled by Aldomet tablets only.
• Therapy to correct specific nutritional deficiency like iron for anemia, vitamin B12 and calcium supplementation.
The selection of patients who received the supplementary therapy was done by simple random sampling. The first case was given N-acetyl cysteine and the second case was not given the supplement but only the conventional medications of PE; which were given for all cases, and so on until 115 cases were obtained; 100 cases completed the study and 15 cases dropped out.
A- Drugs: N-acetylcysteine: "Acetylcysteine sachets" each contains 200 mg acetylcysteine. It is produced by Sedico pharmaceutical Company, 6th October City, Egypt. It was used in a dose of 400 mg/day [8].
B- Chemicals for blood samples digestion before BLL measurement.
All contributors were subjected to:
- Comprehensive history taking: Including personal history, obstetric history and inquiry about environmental exposure to pollutants [10].
- Examination: Pulse and blood pressure: Measured under standardized conditions. Blood pressure is measured while the patient is lying in the left lateral position by mercuric sphygmomanometer, using the 4th Korotkoff phase (K4 = muffling of the sound) for detection of diastolic blood pressure.
- Preeclampsia was defined if there was a rise of blood pressure up to 140/90 mmHg or greater, and appearance of protein in urine with or without edema of the body in pregnant females after 20 weeks' gestation. PE is considered mild if blood pressure rise is up to 140/90 mmHg and proteinuria is ≥ 300 mg/24 hour-urine sample or ≥ 1+ on urinary dipstick test, in pregnant females after 20 weeks' gestation. Severe PE was defined as the rise of blood pressure up to 160/110 mmHg or more and proteinuria ≥ 500 mg/24 hour-urine sample or ≥ 2+ on urinary dipstick test with serum creatinine ≥ 1.2 mg/dl, platelet count < 100,000/cm3, elevated liver enzymes, severe headache, persistent visual disturbances, persistent epigastric pain or severe fetal growth restriction. Superimposed PE was defined as the new onset proteinuria ≥ 300 mg/24 h - urine sample in chronic hypertensive females [11].
- Routine laboratory investigations: Liver function tests, kidney function tests, random blood glucose levels and testing for protein in urine.
- Two milliliters of blood were withdrawn for BLL measurement. Supplementation with 400 mg/day of NAC was initiated together with the specific conventional medications of PE.
- Blood lead levels were re-tested after 4 and 6 weeks of NAC supplementation.
Lead is present in biological samples at low concentrations. Precise and accurate analysis is an important step if meaningful results are to be obtained [2].
Special care was taken during collection process to avoid contamination of the samples with any material whose composition and purity are not known, one milliliter of whole blood was drawn into polyethylene tube; containing heparin as an anticoagulant and stored at -20 ℃ until analysis of lead and trace elements is performed [2].
The ideal material of a container is characterized by law lead impurities and law rate of loss of water vapor. So, high pressure polyethylene and polypropylene tubes are excellent for long period storage of blood samples for lead and trace elements analysis. The ideal duration of storage is 70 days [12].
Lead and trace elements in blood samples are present in a chemically bound form. So, it is essential to destroy the organic matter in the sample and to break the chemical combination of lead and trace elements with the bulk organic substance to obtain a free chemical form of the elements, which can be subsequently determined by the instrument [12].
There are two types of methods that are commonly used for digestion of samples. They are wet digestion and dry digestion. Wet digestion is generally better than dry ashing digestion because it results in lower loss of contents of element in the sample. It is also faster and provides more complete removal of organic matrix [13].
The most commonly used method is acidic oxidation of the sample in an open air system such as beaker at a temperature slightly higher than boiling point of the used acid.
Diluted aqueous perchloric acid, either warm or cold, is not an oxidizing agent. Concentrated perchloric acid (60-72%) is not an oxidizing agent when cold but becomes a powerful oxidizing agent when warm. The danger of the explosion of perchloric acid with organic substance is high, so, it is not used alone for destruction of the organic matter. Perchloric acid is generally mixed with the nitric acid. This allows for a controllable digestion of organic matrix; the nitric acid primarily oxidizes the organic matrix at low temperature (> 120 ℃) before the temperature is sufficient for the perchloric acid to begin the oxidation of the remaining organic substance (a temperature > 160 ℃).
According to Kazi, et al. 2009 [12]:
1. Sample preparation was carried out under clean conditions using deionized water.
2. All chemicals were ultra-pure reagent grade, nitric acid (HNO3) BDH 69% and perchloric acid HCLO3 70%.
3. All glassware and plastic ware were acid-treated (washed three times with deionized water then soaked in 20% HNO3 overnight and then rewashed three times with deionized water and dried until used).
A sample (1 ml whole blood) was pipetted into 50 ml. pyrex beaker then 4 ml. nitric acid were added. A watch glass was placed on the top of the beaker. This mixture was placed on a low heat then temperature was brought slowly to 120 ℃ the point at which nitric acid began to distill. Then, the heating rate was increased gradually. Near dryness, the watch glass was removed and 1 ml. perchloric acid was added and the digestion was continued. It was complete with the appearance of white fumes of perchloric acid. Near dryness, the watch glass was removed, and few milliliters of deionized water were added, and heating was stopped. After cooling, the digestion was completed to 10 ml. by deionized water and stored in 10 ml. acid treated- polyethylene tube [12].
Measurement of lead was performed by a model Perkin-Elmer double beam 2380 Atomic Absorption Spectrophotometry (AAS) that adapted Perkin Elmer hallow-cathode lamps and conventional 10 cm slot burner heat for an air-acetylene flame. The optimum instrumental conditions for determination of lead by AAS are as the following [14] (Table 1).
Table 1: The optimum instrumental conditions for determination of lead by AAS are as the following [14]. View Table 1
Data entry and analyses were performed using SPSS (Statistical Package for Social Sciences) version 16 (SPSS, Inc., Chicago, IL, USA). Qualitative data were presented as number and percentage while, quantitative data were presented as mean, standard deviation and range. The chi-square (χ2) was used to test the association between raw and column variables of qualitative data. The One-Way ANOVA procedure was used to compare means and standard deviations of the studied groups with Post Hoc test for internal comparisons. Paired t-test was conducted to evaluate the impact of time on the mean of variables in the studied groups. Correlation between variables was done using Pearson correlation. P-value of ≤ 0.05 and of ≤ 0.001 indicates significant and highly significant results respectively.
Figure 1 demonstrates BLLs in the studied cases of PE (n = 115) as compared to its levels in the healthy pregnant women (n = 25). There is a highly significant difference between the two groups (P = 0.00).
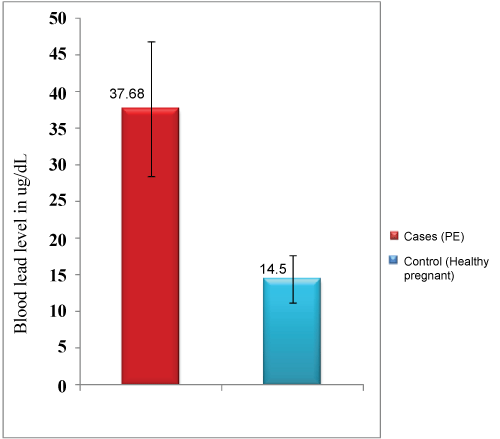 Figure 1: Blood lead levels (mean ± SD) in the study groups (µg/dl: microgram per decilitre; SD: standard deviation). View Figure 1
Figure 1: Blood lead levels (mean ± SD) in the study groups (µg/dl: microgram per decilitre; SD: standard deviation). View Figure 1
Figure 2 and Table 2 demonstrate the BLLs in the two groups of the studied cases at the time of diagnosis and changes in its levels after 4 and 6 weeks of treatment [15].
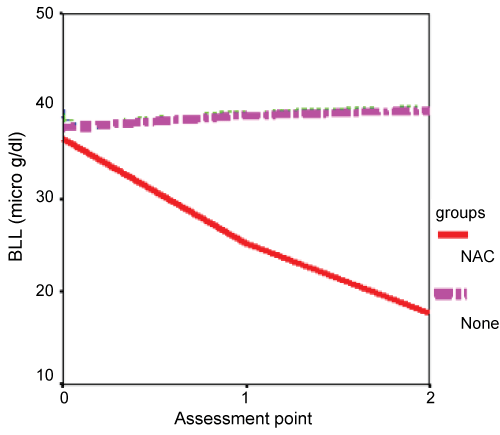 Figure 2: A graph demonstrating the changes in blood lead levels in the two groups of the studied cases overtime of follow up. Note that there was marked decrease of BLL in the group which received NAC supplementation (BLL: blood lead level, NAC: N-acetyl cysteine; µg/dl: microgram per decilitre). View Figure 2
Figure 2: A graph demonstrating the changes in blood lead levels in the two groups of the studied cases overtime of follow up. Note that there was marked decrease of BLL in the group which received NAC supplementation (BLL: blood lead level, NAC: N-acetyl cysteine; µg/dl: microgram per decilitre). View Figure 2
Table 2: BLLs in the two groups of the studied cases at the time of diagnosis and changes in its levels overtime of treatment. View Table 2
It is observed that there was no significant difference between the two groups of PE regarding their initial BLL values, which turned highly significant after initiation of specific supplementary therapy. Note that there was marked decrease of BLL in groups who received NAC supplementation.
Table 3, Table 4 and Table 5 demonstrate the changes in blood pressure and proteinuria in the two groups of the studied cases at the time of diagnosis and after 4 and 6 weeks of treatment.
Table 3: Changes overtime in systolic blood pressure in the two groups of the studied cases. View Table 3
Table 4: Changes overtime in diastolic blood pressure in groups of the studied cases. View Table 4
Table 5: Changes overtime in the degree of proteinuria in the two groups of the studied cases. View Table 5
Table 6, Table 7 and Table 8 demonstrate the correlation between BLLs and cardinal signs of PE at the time of diagnosis and after 4 and 6 weeks of treatment.
Table 6: The correlation between blood lead levels at the time of diagnosis with the clinical indicators in PE cases. View Table 6
Table 7: The correlation between blood lead levels with different clinical indicators in PE cases after 4 weeks of treatment. View Table 7
Table 8: The correlation between blood lead level with different laboratory and clinical indicators after 6 weeks of treatment. View Table 8
Preeclampsia is a complex pregnancy-specific systemic disorder that, despite its great polymorphism, the criteria for its diagnosis have not changed over the past decade (systolic blood pressure > 140 mmHg or diastolic blood pressure ≥ 90 mmHg and 24-hour proteinuria ≥ 0.3 g). Clinical features and laboratory abnormalities define and determine the severity of the disorder [16].
There was a statistically highly significant difference in the blood lead levels in pregnant women suffering from preeclampsia (BLL = 37.68 ± 9.17 µg/dl) as compared to its levels in healthy pregnant women (BLL = 14.5 ± 3.18 µg/dl) (P = 0.00).
This result goes with the results of Motawei, et al. 2013 [2] who found significant positive association between BLL and the elevated blood pressure in pregnant women. Moreover, Rothenberg, et al. 2002 [17] stated that that the lead content in bone affects blood pressure causing hypertension even if the lead level in the blood was within the accepted ranges.
On the other hand, this does not go with the findings of Yao and Huang 2003 [18] who measured BLL in pregnant females to find out the relations between maternal blood lead level and pregnancy complications. They found the mean lead concentration in the maternal blood to be 43.4 µg/l and concluded that the blood lead level is not correlated with pregnancy complications or neonatal physical and neurobehavioral development, but it is negatively correlated with the maternal hemoglobin concentration.
This can be explained by the relatively low BLL in this study (4.34 µg/dl). At this level, lead affects haemoglobin synthesis and cause anaemia, while lead effects on blood pressure and neonatal physical and neurobehavioral development appear at higher levels of the toxic metal in the blood and tissues.
There is an evidence of a causal relationship between lead exposure and hypertension [19]. A study related bone lead with blood pressure in a cohort of 575 men indicated that an increase in the incidence of hypertension among individuals with elevated bone lead content [20]. The CDC stated that the lead-induced elevated blood pressure is more pronounced in the middle age than in the young age [21].
It is to be noted that the level of lead in blood of the control group of the healthy pregnant women in this study is higher than the accepted range in many countries.
In USA, strict lead exposure prevention strategies had succeeded to decrease BLL in population to extraordinary small values [10].
Strict exposure guidelines developed by the U.S. Centers for Disease Control and Prevention (CDC) and the American Pediatric Association consider blood levels ≥ 5 μg/dl to be excessive for infants, children, and women of childbearing age. Occupational exposure is unsafe when worker's blood levels exceed 25 μg/dl [21]. However, Kim, et al. believe that there is no safe level for lead exposure as the low-level exposure to Pb still shows severe toxicity in high susceptible population and late onset of some diseases from early exposure [22].
The remarkably high blood Pb levels found in patients and subjects of this study may be attributed to the high prevalence of environmental pollution in the developing countries including Egypt [2,3].
Also, the mobilization of lead from bone during pregnancy leads to a remarkable gestational rise in maternal blood lead levels that can pass through the placenta to the fetal skeleton [23].
Motawei, et al. 2013 [2] have studied different demographic and environmental data to find an explanation to this remarkably high blood lead level in the cases of preeclampsia. These included the age, residence, occupation, husband occupation, busy street residence, passive smoking, use of traditional cosmetics and kohl, vitamin therapy, building material, house remodelling, use of canned food, use of lead-glazed ceramics and pottery and use of herbal medical products and pica. All the data were insignificant between the preeclampsia and the control group except the husband occupation.
This confirms the relation between the occupation and the risk of lead exposure. The at-risk husbands' occupations may cause elevated blood lead levels in the pregnant women through lead particles in the husbands' clothes that they carry to homes and the family members become exposed to.
The investigators also found that the following socio-demographic factors were significantly related to the elevated BLLs within the PE patients: the age, vitamin therapy and the use of lead-glazed ceramics and pottery [2].
The difference between blood lead levels between the two groups before treatment was insignificant (P = 0.42). It tuned highly significant after supplementation with NAC (P = 0.00).
This denotes the ability of N-acetyl cysteine to lower lead levels in the blood. This agrees with the results of Wang, et al. [24] who found that administration of NAC to experimental animals, leads to significant increase in urinary Pb excretion and decrease in Pb levels in the blood and the manifestations of toxicity in the animal. The investigators explained these findings by the potent ability of N-acetylcystein to scavenge reactive oxygen species (ROS) which play an important role in lead toxicity.
Polyak, et al. [25] and Zhang, et al. [26] agree to the same concept; that NAC can protect against toxins-induced apoptosis and can protect against oxidative stress in mitochondrial complex I diseases.
Motawei, et al. [27] found an improvement in the oxidative stress status and a decrease in hypertension and the degree of proteinuria in preeclampsia women who received NAC supplementation.
Li and Fu 2000 [28] stated that the neurotoxic effects of lead on developing brains are related to the imbalance in redox status which is mainly mediated by decrease of GSH content secondary to lead exposure. The investigators also stated that N-acetyl cysteine replenishes glutathione stores and shifts the balance in favor of antioxidants, hence its beneficial effects in prevention and treatment of manifestations of lead toxicity.
Nehru and Kanwar, 2007 [29] and Pedroso, et al. [30] agreed to the same opinion that NAC can be useful in arresting the neuro-toxicological damage of lead in cerebral and cerebellar brain regions through the effects of NAC on glutathione (GSH) status.
There were no significant differences between different groups of the study in their initial blood pressure values, either systolic or diastolic BP (P = 0.88 and 0.42) (Table 3 and Table 4). The differences turned significant in the second and third follow up times (P = 0.03 and 0.00). The differences were highly significant overtime in each group (P = 0.02, 0.01, 0.00).
These results indicate that antioxidants can help in decreasing the elevated BP in preeclampsia particularly if given for a longer period (one month or more) with the conventional antihypertensive medications.
Regarding the degree of proteinuria, there was no significant difference between the two groups of the study initially (P = 0.87), then it turned significant and highly significant after 4 and 6 weeks respectively (P = 0.02 & 0.00 respectively) (Table 5).
Table 6, Table 7 and Table 8 show significant correlations between BLLs in the cases and the severity of the clinical manifestations of PE on diagnosis and over time of follow up.
These results agree with Nawathe and David [31] who believed that NAC Melatonin and creatine are novel neuroprotective and cardioprotective agents in conditions of poor placentation and fetal growth restriction, like that which happen in preeclampsia.
On the other hand, Roes, et al., 2006 [32] stated that antioxidants cannot stop the process of already established PE.
This may be explained by the fact that preeclampsia is a multi-factorial disease, and oxidative stress is one factor of PE. Lowering oxidative stress status and elevating antioxidant reserve in women with poor antioxidant status would be beneficial in ameliorating the severity of PE and decreasing its organ-damaging sequelae [33].
Blood lead levels in pregnant females suffering from PE was significantly higher than its level in the healthy control pregnant females (BLL was 37.68 ± 9.17 µg/dl in PE cases versus 14.5 ± 3.18 µg/dl in healthy pregnant control group). The difference between the two groups was highly significant (P-value = 0.00).
None of the risk factors for lead exposure was significant between the two groups except the factor of husband occupation.
The difference in BLL between groups of patients with PE was initially insignificant that turned highly significant after initiation of the supplementary therapy.
The same was observed regarding the manifestations of PE including blood pressure and proteinuria.
Despite improvement of the clinical manifestations of PE, the disease process did not stop. This suggests that oxidative stress is a consequence not a cause of PE.
• Lead is closely related to preeclampsia/pregnancy hypertension.
• N-acetylcysteine is a powerful lead-chelating agent that is the particularly useful in pregnancy.
• Lead screening may be a useful predictor of hypertension/preeclampsia particularly in women with a history of previous hypertensive disorders in pregnancy.
• N-acetyl cysteine has more useful lead-chelating effect when used for at least one month.
None.