Amniotic fluid embolism (AFE) is a rare but life-threatening obstetric emergency, often challenging to diagnose due to overlapping clinical presentations. We present a case of AFE-induced cardiovascular collapse and disseminated intravascular coagulopathy (DIC) in a 38-year-old patient G6P4A1V3, emphasizing the critical role of a multidisciplinary approach and the importance of timely recognition in successful management.
Amniotic fluid embolism, Cardiac arrest, Disseminated intravascular coagulation, Hysterectomy, Postpartum period, Case report
Amniotic fluid embolism (AFE) is a rare-estimated incidence 1.9-6.1 per 100,000 pregnancies [1]- but life-threatening condition with maternal mortality rates ranging from 10 to 90%. While prognosis rely on early recognition and assessment, diagnosis is often retrospective due to overlapping clinical presentations. We describe a case of AFE-induced cardiovascular collapse and disseminated intravascular coagulopathy (DIC) in a 38-year-old patient. She underwent subtotal hysterectomy and was successfully managed with a multidisciplinary approach.
The patient was a 38-year-old G6P4A1V3, immigrated from Côte d’Ivoire 7 years prior, with a past history of chronic anemia and prior unrecognized hepatitis B infection. Her obstetrical history revealed a term stillbirth, followed by two uncomplicated vaginal births, a C-section for breech presentation and medical induced abortion 2 years prior the actual pregnancy.
Apart from chronic anemia treated with 3 doses of IV iron during the third trimester, her pregnancy progressed uneventfully. She presented at 39 3/7 weeks gestation for decreased fetal movement. Routine assessment showed normal fetal wellbeing but given her stillborn history, induction of labor with prior cervical ripening by balloon catheter was immediately scheduled. She was admitted to Labor and Delivery unit at a tertiary hospital by the family medicine team.
The patient’s labor progressed uneventfully. Fetal heart rate showed uncomplicated variable decelerations in late first stage and second stage, though no instrumentation was required. She had vaginal delivery of a 3 kg female baby, Apgar scores 4-7-10 due to initial respiratory distress. Spontaneous delivery of the placenta occurred within 15 minutes and first-degree laceration was repaired. The patient developed a postpartum hemorrhage, with 500 mL estimated blood loss. Initially, she received oxytocin infusion and oral misoprostol. The uterine revision was negative. Vitals were stable. As bleeding persisted over the next hour, she received a total of 4 doses of carboprost tromethamine, 1g of IV tranexamic acid and a single dose of methylergonovine. Repeat uterine revision was unremarkable. The maternal fetal medicine (MFM) team was called given poor response to uterotonic regime. Reassessment showed no signs of placental retention, uterine rupture nor additional vaginal/cervical lacerations. Bleeding slowly decreased with uterotonic drugs, added sutures to the perineum and constant compression. Blood loss was then estimated at 1500 mL and bloodwork revealed a drop in hemoglobin concentration from 112 to 95 mg. Unfortunately, the coagulation bloodwork were only available 3 hours and 20 minutes after the delivery because the first sample was hemolyzed. Transfusion of a single unit of packed red-cells was initiated based on the hemoglobin drop.
Approximatively two hours and half after the placental delivery, the MFM team was called back to bedside for recurrent bleeding and heavy pain. Anesthesia was optimized before Bakri balloon tamponade was attempted, but unsuccessful. During that timeframe, the patient had hypotension, tachycardia and decreased level of consciousness. Based on the possibility of an intra-abdominal hemorrhage and acute hemodynamic changes, the patient was transferred to the operating room. The gynaecology team was called for surgical assistance. Preoperative blood loss was then estimated at 2500 mL. The institutional massive hemorrhage protocol was initiated before surgery, while hemodynamic support and ventilation was managed by the anesthesiology team. At this point, the patient was anuric and partially responsive to fluids and vasopressors. Intraoperative findings included no intra-abdominal bleeding and persistent moderate vaginal bleeding. A decision was made to proceed to emergency subtotal hysterectomy, while post-partum hemorrhage still appeared as the main cause of shock. Bloodwork available at the beginning of the hysterectomy showed severe metabolic acidosis, hemoglobin concentration of 80 mg/L, platelet count of 133 × 10 9 /L, INR of 2,3, APTT of 86.5 seconds and plasma fibrinogen level of 0.32 g/L, confirming the disseminated intravascular coagulopathy status.
At the beginning of the procedure, the patient developed ventricular tachycardia and went into cardiorespiratory arrest. Cardiopulmonary resuscitation was immediately performed and she required defibrillation. Perioperative consultation to cardiology was inquired. Bedside transthoracic echocardiogram revealed pulmonary hypertension, right ventricular dilatation and hypokinesis, preserved left ventricular ejection fraction, right atrial dilatation and absence of clots. Perioperative laboratory values showed a serum lactate of 12 mmol/L, thrombocytopenia, APTT of 93 seconds and plasma fibrinogen level of 0.56 g/L. With standard acute cardiovascular life support, the patient was stabilized and remained stable throughout the surgery. The patient stayed in the operating room for four hours and underwent subtotal hysterectomy with unilateral salpingectomy and ureteral catheterization. Total perioperative blood loss was estimated at 2500 mL. The patient received a total of 9 units of packed red-cells, 7 units of frozen plasma, 4 packs of cryoprecipitate and 1 unit of platelets.
Pathologic examination of the surgical specimen confirmed multiple amniotic fluid embolic material inside the uterine vessels (Figure 1).
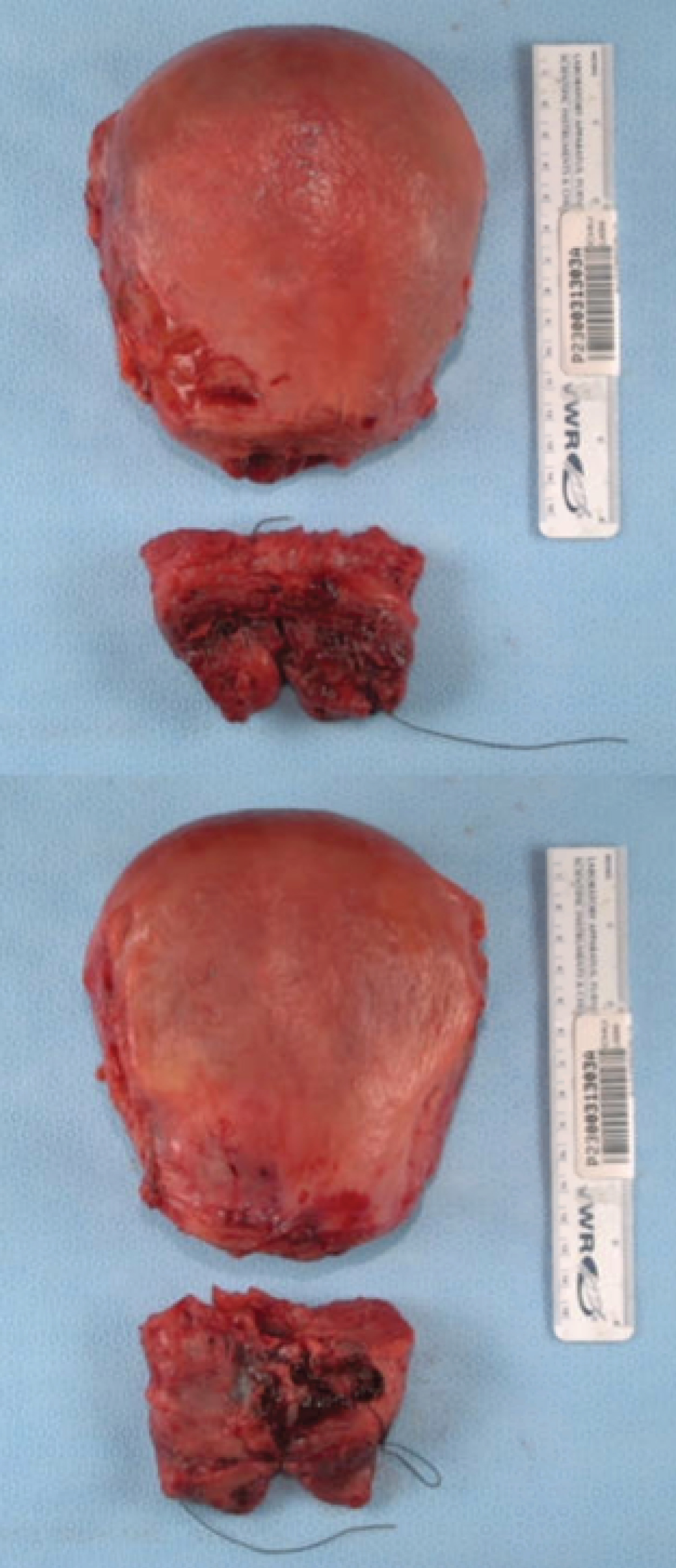 Figure 1: Surgical specimen. Macroscopic view of the patient's uterine body.
View Figure 1
Figure 1: Surgical specimen. Macroscopic view of the patient's uterine body.
View Figure 1
Postoperative CT pulmonary angiogram showed extensive bilateral pulmonary embolism with signs of right heart failure. The patient was transferred to a tertiary cardiology and pneumology university center on the same day. She underwent mechanical thrombectomy, angioembolization of the AV fistula and insertion of an inferior vena cava filter. Even if the immediate postoperative CT pulmonary angiogram showed extortive bilateral pulmonary embolism the radio-interventionist concluded that there was only a small residual clot to perform the thrombectomy. She was extubated 3 days after her surgery. She required 4 days of continuous Veno-Venous hemofiltration and 1 dialysis treatment for acute kidney surgery.
The patient had an uneventful recovery and was discharged two weeks later with therapeutic dose of warfarin given her desire to breastfeed. She showed no sign of neurological nor renal adverse outcome. At her routine postoperative visit two months postpartum, clinical and physical examination were as expected. Assessment for underlying thrombophilia was conducted by an obstetric internist, only revealing a slightly positive lupus anticoagulant.
AFE is a rare yet potentially life-threatening condition. While the pathophysiology remains poorly understood, the low incidence complicates the establishment of a consensus on risks factors and diagnosis criteria significant enough to modify standard obstetric care. In a national registry [2], 70% of AFE cases occurred during labor, 11% after vaginal delivery and 19% during caesarian section. In the absence of universal serological or pathological markers, AFE is diagnosed clinically, based upon the exclusion of other potential etiologies and the presence of clinical findings, such as sudden respiratory distress (hypoxia), circulatory collapse (hypotension) and/or seizures, often followed by disseminated intravascular coagulopathy (DIC). However, these characteristic findings are not present in all cases of AFE, confounding clinical suspicion. Regardless, most patients present a prompt and drastically evolutive state, underscoring the critical importance of timely recognition for prognosis.
Diagnostic criteria for AFE were proposed by the society for maternal-fetal medicine (SMFM) [3], all of which must be present: (1) Sudden onset of cardiorespiratory arrest or hypotension with evidence of respiratory compromise; (2) Documented DIC; (3) Absence of fever during labor; (4) Clinical onset during labor or within 30 minutes of placental delivery. Although meant for research purposes, these findings can also be of clinical use, whereas DIC serves as the hallmark laboratory finding in the differentiation between AFE and other conditions with overlapping presentations. It is also in line with previous studies that reported DIC occurring in up to 83% of cases of AFE [2].
In this case, the patient had almost all the AFE criteria, except the time frame onset. Upon reflection, an expedited second evaluation of DIC retrospectively emerges as a critical consideration that could have potentially streamlined the management process. As advised by the SMFM clinical guidelines on diagnosis and management in AFE [4], the identification of DIC markers could have served as an early indicator, prompting a more targeted intervention.
The coordinated efforts of multiple specialties, as observed in this case, played a pivotal role in achieving stabilization. Effective communication and collaboration between the obstetric, anesthesiology, cardiology and gynecology teams contributed to the timely and effective management of the patient, highlighting the essential role of a synchronized and cooperative approach in navigating the complexities of uncertain diagnostic scenarios. This also shed light on the necessity of simulation-based training for such critical, although rare, clinical events. The successful outcome in this case is a testament to the synergistic collaboration among healthcare professionals in the face of obstetric emergencies, reinforcing the critical role of teamwork in optimizing patient care.
This case serves as an illustration of the inherent urgency in managing AFE, underscoring the imperative for rapid decision-making and the essential role of interdisciplinary teamwork. The urgency emanates from the unpredictable and potentially life-threatening nature of AFE, demanding immediate and well-coordinated responses from diverse medical specialties.
The woman whose story is told in this case report has provided written consent for its publication.
The authors wish to thank Dr. Dany Côté (Department of Anesthesiology), Dr. Stéphane Côté (Department of Obstetrical Medicine), Dr. Patrick Couture (Department of Critical Care Medicine), Dr. Catherine Dagenais (Department of Maternal Fetal Medecine), Dr. Francesca Donders (Department of Gynaecology), Dr. Marie-Hélène D’Amours (Department of Family Medecine), Dr. Mario Langlais (Department of Cardiology), Dr. Pascale Ouellet (Department of Anesthesiology) and Dr. Katherine Thériault (Department of Maternal Fetal Medecine) from Centre hospitalier de l’Université Laval (CHU de Québec-CHUL). We also wish to extend appreciation to Dr. Judith Bellemare (Department of Nephrology), Dr. François Dubé (Department of Internal Medicine) and Dr. Stephan Langevin (Department of Critical Care Medicine) from Institut Universitaire de cardiologie et de pneumologie de Québec (IUCPQ).
The authors report no conflict of interest.
All authors meet the criteria for authorship.