Uterine rupture is a disruption of the uterine scar, causing foetal expulsion into the peritoneal cavity. This condition, primarily caused by the separation of uterine scar tissue from previous caesarean surgery, reduces foetal survival and increases maternal morbidity and mortality. A 32-year-old woman with a history of four vaginal deliveries and one caesarean section was diagnosed with uterine rupture, leading to a laparotomy procedure and obstetric hysterectomy. Consistent antenatal care can prevent uterine rupture.
Uterine rupture, Postpartum haemorrhage, Hysterectomy
A rupture of the uterus is characterized by a complete separation of the uterine wall from the serosa that lies above. Severe morbidity and mortality in both the mother and the newborn are associated with this uncommon peripartum complication [1]. A complete uterine rupture occurs when there is a direct communication between the amnion and peritoneal cavity, primarily due to uterine scar dehiscence from a previous caesarean section. Overstimulation of oxytocin can cause uterine rupture, but this is now rare. The reported mortality rate attributed to uterine rupture ranged from 50% to 75% [2]. A rupture is least likely to occur in an unscarred uterus. Several conditions have been identified as potential predisposing factors for uterine rupture, including grand multiparity, neglected labour, malpresentation, breech extraction and uterine instrumentation [3]. Uterine rupture can lead to foetal and maternal outcomes, depending on the duration between diagnosis and birth. Foetal outcomes may include admission to the neonatal intensive care unit, hypoxia, and neonatal mortality. Maternal outcomes may include haemorrhage, hypovolemic shock, bladder injury, hysterectomy, and maternal mortality. The morbidity and death rates depend on the quality of medical care provided [4].
Obstetricians must have a thorough understanding of uterine rupture to provide timely interventions, preventing adverse outcomes for both the mother and the foetus.
A 32-year-old woman, P5L4D1, was referred to our institute for further management of traumatic PPH after delivery. Her obstetric history includes two full-term normal vaginal deliveries 12 and 8 years ago, a caesarean section five years ago, a vaginal birth after caesarean (VBAC) two years after, and a second vaginal delivery after a caesarean section. The patient was conscious, afebrile, and had a pulse rate of 140/min and a blood pressure of 90/60 mmHg. She had signs of dehydration and pallor, abdominal tenderness, and abdominal distension. A cervical tear was found during a vaginal examination. Ultrasonography revealed the lower body of the uterus and cervical margins were not delineated. Hemoperitoneum was found in the pelvis, hepatorenal pouch, and peri-splenic region. Preoperative laboratory tests revealed an abnormal coagulation profile and haemoglobin of 6.2 g/dL. The diagnosis of uterine rupture was established based on the clinical findings and ultrasound report. Following the administration of prompt resuscitative interventions, the patient was subsequently taken up for laparotomy. Prior to performing the laparotomy, the attendant acquired informed consent. Two units of platelets, four units of fresh frozen plasma (FFP) and four units of packed red blood cells (PRBC) were also arranged to be administered. An emergency laparotomy was performed under general anaesthesia with utmost adherence to standard operating procedures. A tear that extended lower to the cervix and deep into the vagina was visible, indicating that the previous uterine scar had given way (Figure 1).
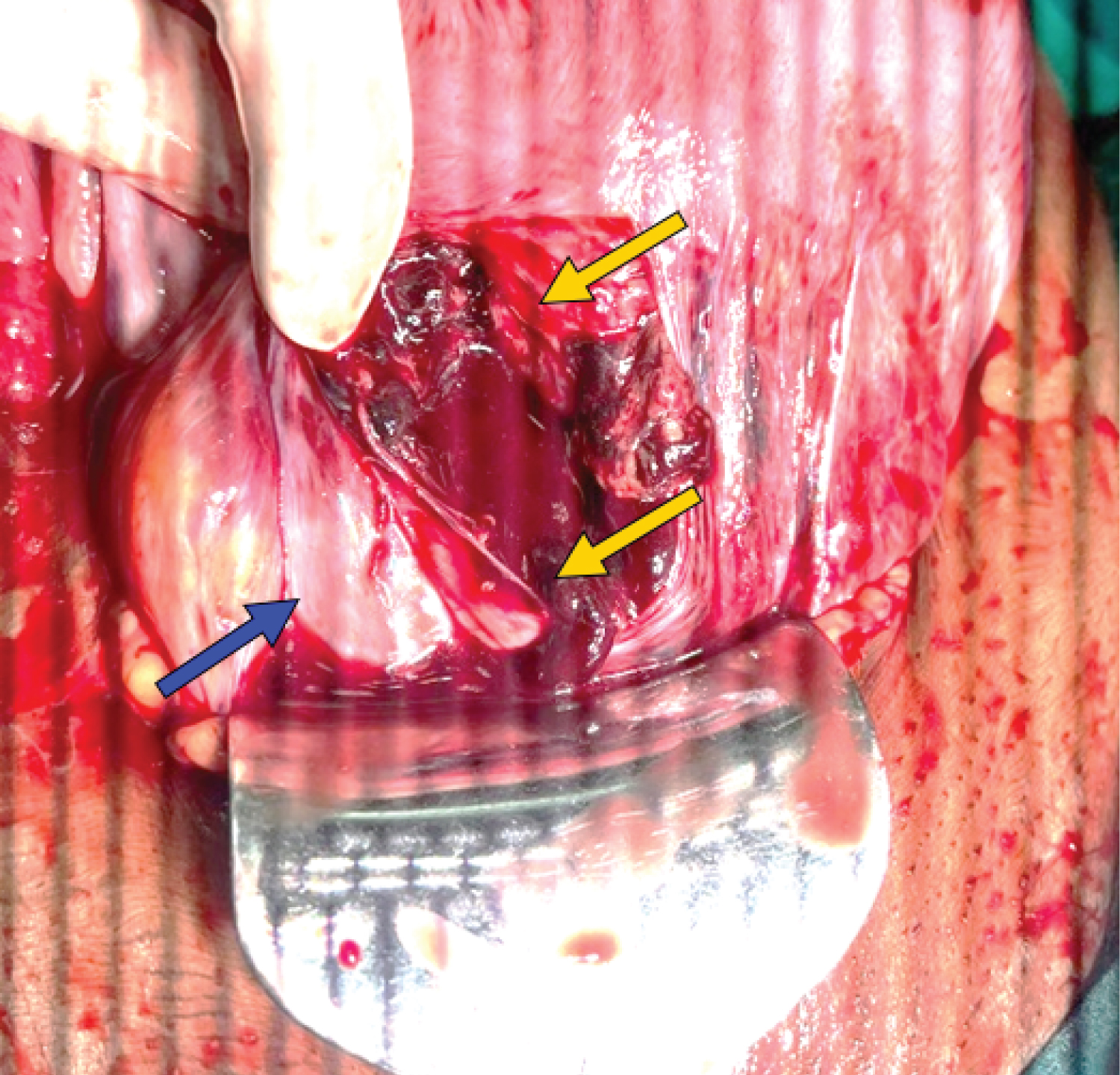 Figure 1: Uterine scar rupture extending below to cervix and deep into the vagina (yellow arrows); Bladder intact (blue arrow).
View Figure 1
Figure 1: Uterine scar rupture extending below to cervix and deep into the vagina (yellow arrows); Bladder intact (blue arrow).
View Figure 1
The left uterine artery was involved, causing significant bleeding. It was promptly clamped. The bladder was dissected and its structural integrity was preserved, as confirmed by retrograde filling with methylene blue. A total abdominal hysterectomy procedure was performed, during which complete haemostasis was successfully obtained. Following this, a drain was inserted and the abdomen was closed in layers after completing a mop and instrument count.
Following surgery, the patient received six units of PRBC, four units of FFP, and two units of platelets in the obstetric intensive care unit (ICU). They were given inotropic support until the third surgical day, then weaned off. Drain was removed and dressing was done on the fifth postoperative day. The sutures were removed on the tenth postoperative day, the urine catheter was removed on the 14 th , and the patient was discharged in good condition on the fifteenth postoperative day.
A rupture of the uterus occurs when the uterine wall separates from the serosa, leading to significant maternal and neonatal morbidity and mortality. The most common risk factor is a prior caesarean section, with a 1% incidence. The World Health Organization estimates that unscarred uterine ruptures occur in 0.006% of births, ranging from 1/8000 to 15,000 births [1-3,5]. Uterine rupture in primigravid patients is rare and often unexpected. The incidence is higher in developing countries due to poor antenatal and obstetric care, high frequency of home deliveries with prolonged labour, and grand multiparity, which can lead to premature caesarean sections and in-time caesarean sections [5,6].
Effective management of hypovolemia and bleeding control are critical for the survival of expectant women who have experienced uterine rupture [7]. Management is determined by the patient's general condition, the site and type of rupture (complete or incomplete), the amount of haemorrhage, the age and number of healthy children, and the obstetrician's capabilities [2]. For emergency caesarean sections, the Royal College of Obstetricians and Gynaecologists (RCOG) advises a 30-minute decision-to-delivery time interval. Severe hypoxia, metabolic acidosis, and other severe neonatal complications may still occur despite attempts to deliver the foetus within this timeframe. Foetal or placental extrusion through the uterine wall is likely to result in irreversible foetal injury prior to the anticipated time of delivery. Uterine rupture can be averted through meticulous assessment, particularly in the context of prenatal care (Table 1) [8].
Table 1: Warning signs of an impending uterine rupture [8]. View Table 1
The RCOG recommends that women with a previous caesarean section undergo antenatal care on five occasions: At 12 weeks, 18-21 weeks, 21-28 weeks, 32-34 weeks, and 36 weeks. Additionally, it is crucial to schedule three visits to an obstetrician, beginning with visits at 12 weeks of gestation, in order to ascertain the most suitable method of delivery [8]. The obstetrician should conduct a thorough examination regarding the lower uterine segment thickness and medical history [9].
A ruptured uterus has detrimental effects on both the survival of the foetus and the mother. An expectant woman presenting with acute and severe abdominal pain should be examined with a high degree of suspicion for uterine rupture. Prompt diagnosis and treatment are critical in the event of a uterine rupture. Prenatal care, including obstetric visits, can prevent uterine rupture by assessing the foetus and mother's condition and determining the most effective delivery method.
None.
None.
None.