Background: Listeriosis is a rare and severe foodborne infection caused by Listeria monocytogenes, which manifests as septicemia and central nervous system (CNS) infections with a high fatality rate of around 20 to 30%. However, neonatal listeriosis has rarely been reported in UAE.
Case presentation: We describe a case of Neonate female who was born 32 weeks gestational age via emergency LSCS due to fetal decelerations. Mother is a G2P1A0 who had a history of fever 4 days prior to delivery. Birth weight 1.9 kilograms. APGAR 7, 8 and 9, at 1st, 5th and 10th minute of life, respectively. Patient required CPAP at birth in view of respiratory distress. Blood culture collected on the 1st day yielded Listeria monocytogenes.
Conclusion: Listeria infections should be considered in infants who present with erythematous rash, respiratory distress and nonspecific findings of early neonatal sepsis. Although an empirical antibiotic therapy including ampicillin and aminoglycoside combination is effective by the means of a probable Listeria infection, the progression of the very early-onset disease may be fatal despite vigorous treatment efforts.
Listeriosis, Listeria monocytogenes, Neonate
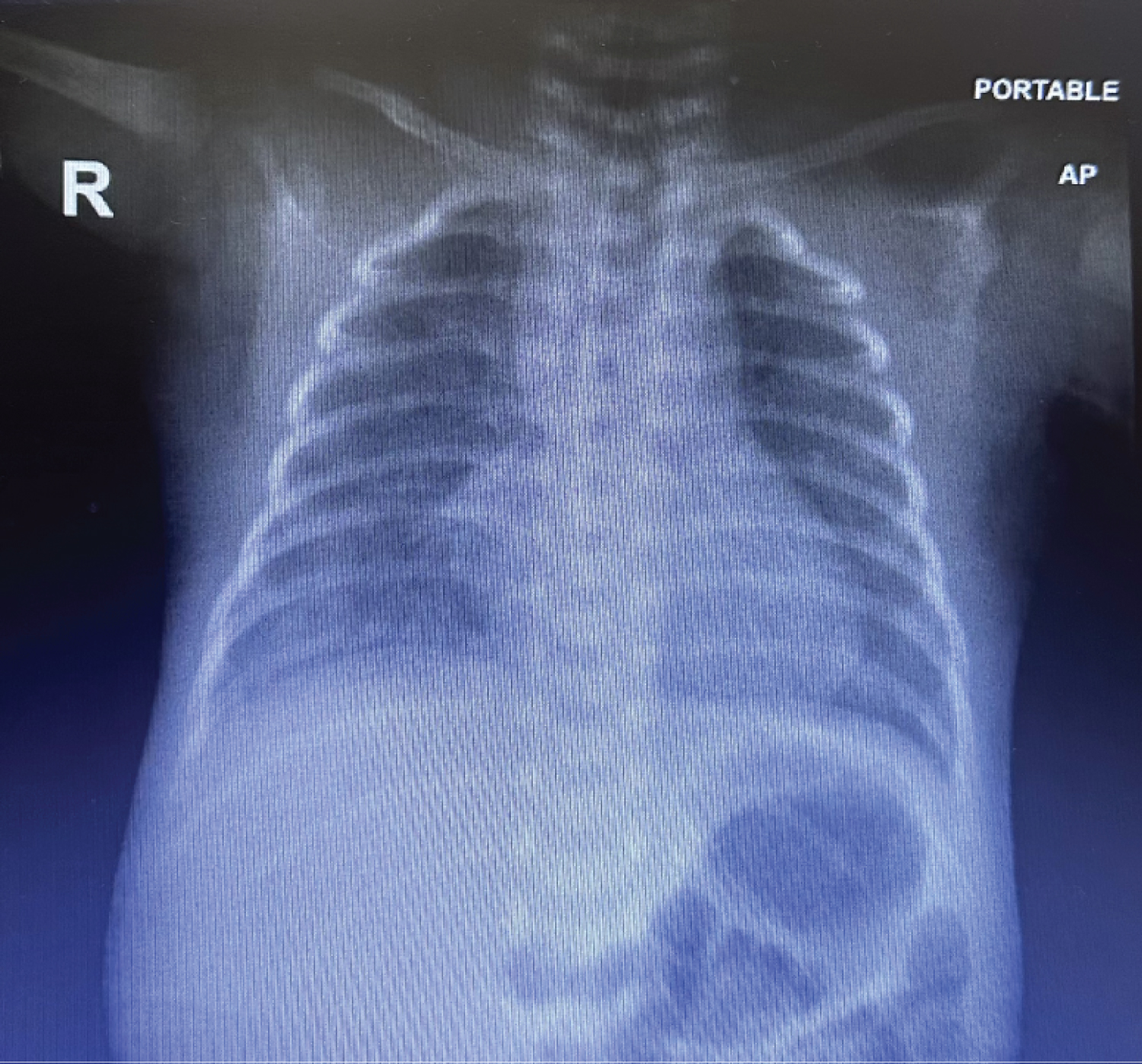 Figure 1: CXR: Increased lung markings in both lungs with mild haziness in both lower zones, more in right side.
View Figure 1
Figure 1: CXR: Increased lung markings in both lungs with mild haziness in both lower zones, more in right side.
View Figure 1
Listeria (L.) monocytogenes is a Gram-positive foodborne pathogen which is often found in diverse environments such as soil, water, various food products, animals, and humans [1]. Neonatal listeriosis is an infection caused by Listeria monocytogenes, leading to sepsis and meningitis with a high fatality rate around 20 to 30% [1,2]. Early-onset neonatal listeriosis (defined as infection in neonates with less than 7 days of age) is mostly caused by vertical transmission from the mother [3,4].
Pregnant women have 12-fold increased risk than normal population to acquire listeriosis after consumption of contaminated food [5]. L. monocytogenes can traverse the placenta and infect the fetus, resulting in abortion, stillbirth, premature birth and neonatal listeriosis [6]. However, maternal listeriosis is relatively mild in severity manifesting as flu-like symptoms (such as fever, myalgia, nausea and vomiting) with 30% of maternal listeriosis patients being asymptomatic [1]. Thus, it is difficult to recognize maternal-neonatal listeriosis due to the mild or asymptomatic maternal listeriosis before it develops into life threatening conditions of neonatal bacteremia or meningitis through vertical transmission. While neonatal sepsis and meningitis cases caused by L. monocytogenes are relatively rare (2 to 7% of infections) [7,8], it is vital to increase clinicians’ awareness on maternal-neonatal listeriosis for early diagnosis and effective targeted treatment, especially in countries where listeriosis is less recognized and there are no surveillance systems in place [9]. We illustrate through a case report of early neonatal sepsis, the importance of the identification of Listeria monocytogenes and its therapeutic implications.
A female preterm neonate weighing 1900g was born via cesarean section due to fetal distress at a gestational age of 32 weeks. Mother is a G2P1A0 who had a history of fever 4 days prior to delivery. In the laboratory investigation of the mother, C reactive protein of 29 mg/L (High) was detected, she was started an empirical treatment of ceftriaxone and metronidazole after obtaining a blood culture. At delivery, meconium-stained amniotic fluid was noted. APGAR 7, 8 and 9, at 1 st , 5 th and 10 th minute of life, respectively. In view of respiratory distress, patient was started on CPAP PEEP 6-7 cm water then transferred to the neonatal intensive care unit (NICU). Physical examination on admission revealed mildly depressed activity, mild generalized edema and poor peripheral perfusion, tachypnea, and subcostal retractions.
Chest Radiography showed: Increased lung markings in both lungs with mild haziness in both lower zones, more in right side. Blood pressure was within normal limits. In whole blood count analysis hemoglobin, leukocyte and platelet counts were 11.5 g/dl, 8834/mm 3 and 176000/mm, respectively. C-reactive protein was 24 mg/L. Then increased over 18 hrs to 42 mg/L.
An empirical antibiotic treatment with ampicillin (100 mg/kg/day q12h) and Gentamycin (4 mg/kg/day q36h) was started initially for neonatal sepsis after a blood culture was sent to the microbiology laboratory. Blood culture collected on the 1 st day yielded Listeria monocytogenes .
CSF culture showed no growth and negative PCR multiplex, C-reactive protein levels were monitored and decrease to normal level by day 4 of treatment. A repeat blood culture was done after 72 hrs of treatment which showed no growth at 48 th hour and 5 th day of incubation period. Baby received full course 10 days of antibiotics then discharged home in good condition to be seen in neonatology follow up clinic.
monocytogenes is an underdiagnosed and under-reported cause of congenital sepsis [10]. The incidence of maternal-neonatal listeriosis, defined by listeria in any sample of maternal, fetal, or neonatal origin. In pregnant women has been estimated at 12 per 100,000, compared with 0.7 per 100,000 in the general population. In Europe and North America, 4-10/100,000 pregnant women/year are described; it constitutes a mandatory reporting disease [10,11]. In underdeveloped countries, no records are kept, but sporadic cases or outbreaks are described [12]. In some studies, pregnant women accounted for 7% of hospitalizations for listeriosis, and neonatal infections accounted for 4% [13].
Neonatal infection It is mainly transmitted via the placenta and through the birth canal or through a nosocomial route. The clinical characteristics of neonatal listeriosis are similar to the neonatal Group B streptococcus sepsis, with early and late form of disease. The early onset disease presents within the first 5 days of delivery, especially in the preterm infants of symptomatic mothers. The late onset disease presents during the 1 st to 4 th weeks of life, usually as a purulent meningitis [7]. The mother generally remains asymptomatic.
The most common signs and symptoms in order of frequency in early listeriosis are respiratory distress (cyanosis, apnea, retractions), fever, neurological abnormalities (lethargy, seizures), rash (maculopapular or papulovesicular), and jaundice. Less common is focal necrosis in the liver, spleen, lungs, and intestines) [11,14]. The presence of meconium or brown amniotic fluid has been described in premature infants with listeriosis [15,16].
Diagnosis in the neonate by the presence of the Gram stain of a gram-positive bacillus in a sample of amniotic fluid, other fluids, or skin lesions helps in the presumptive diagnosis. The presence of monocytosis in the blood or the cerebrospinal fluid is not a defining or valuable characteristic [17]. The definitive diagnosis is established mainly by blood culture, followed by positivity in the cerebrospinal fluid culture; the polymerase chain reaction in cerebrospinal fluid is sensitive and specific for the diagnosis of meningitis.
Neonatal Listeriosis, clinically, it can present as pneumonia, bacteremia/sepsis, or meningitis, the latter being the leading cause of death; at a lower gestational age, mortality is higher, or patients survive with neurological dysfunction [11,16]. Ampicillin continues to be the empirical antibiotic of choice in newborns; if there is no central nervous system involvement, a duration of 10 to 14 days is considered adequate, and in meningitis, 14 to 21 days. Synergism with aminoglycosides is recommended in various guidelines; the results in the studies are diverse, some report a reduction in mortality, and others have not found a beneficial effect. It is essential to consider resistance to cephalosporins, vancomycin, and less effective carbapenems, piperacillin/tazobactam [16-18].
Our case represents an early-onset neonatal sepsis syndrome that is associated with prematurity and pneumonia. Maternal blood remained sterile after routine incubation, the placenta and amniotic fluid were not sampled, Vaginal swab was negative. This supports the evidence of poor correlation between positive maternal blood culture and positive fetal blood culture [19]. A case of early-onset neonatal listeriosis with negative maternal blood culture has been reported before [20].
False determination of culture results due to its coccobacilli shape and changeable Gram-positive stain may be one of the impacting factors in its incidence detected lower than expected. Obstetricians rarely examine materials and take cultures from placenta and fetus in abortus, which may also result in misdiagnosis of perinatal infection, and thus many cases of neonatal listeriosis may remain as undiagnosed. It is, thus, difficult to calculate the exact prevalence of perinatal infections [20].
Maternal-neonatal transmission listeriosis is a rare infection with a severe presentation in this age group and is underdiagnosed in developing countries. We wish to emphasize the necessity of considering listeriosis as a part of differential diagnosis of neonatal septicemia or a clinical scenario when a pregnant woman presents with fever associated with non-specific complaints, especially in third trimester. As in our case the neonatal recovery from septicemia was attributed to an early recognition of the organism, prompt liaison of microbiologist with neonatologist and administration of recommended antibiotics. The progression of the very early-onset disease may be fatal despite vigorous treatment efforts.
Not declared.
WA collected the details, consents and images. MO was responsible for the manuscript.
Not received.
Data were collected from medical files and are not publicly available due to patient confidentiality but are available through the corresponding author under clearly justified academic requests.
Not applicable.
Written informed consent was obtained from the parents of the newborn for publication of this case and the accompanying Images. A copy of the written consent is available for review.
The authors declare that they have no competing interests.