Left ventricular noncompaction cardiomyopathy (LVNC) is a rare form of familial cardiomyopathy attributed to failure of ventricular compaction during embryogenesis. Scant data exists regarding the management approach to pregnant patients with this condition. Additionally, few cases are reported in which both the mother and fetus are simultaneously affected by this condition during the antepartum period. This case is unique in that the patient carried a known genetic and echocardiographic diagnosis of LVNC, prior children born with confirmed LVNC by genetic and echocardiographic findings, and the current pregnancy affected by a perinatal diagnosis of LVNC via sonographic findings.
Cardiomyopathy, Noncompaction, Prenatal diagnosis, Ebstein, MYH7
LVNC: Left Ventricular Noncompaction Cardiomyopathy; VSD: Ventricular Septal Defect; LVEF: Left Ventricular Ejection Fraction
Left ventricular noncompaction cardiomyopathy (LVNC) is a rare form of predominantly familial cardiomyopathy attributed to failure of ventricular compaction during embryogenesis [1,2]. It is characterized by a prominent trabecular meshwork and deep intra-trabecular myocardial crypts located principally near the apex of either or both ventricles which extend in communication with the ventricular cavity. This results in thickened myocardium with two layers - compacted and non-compacted [2,3]. The recesses are extensions into the left ventricular cavity and are filled with blood that is not in communication to the epicardial coronary artery system [3,4]. While it can occur in both ventricles, it is more commonly seen in the left. Typically, the left ventricle is dilated and demonstrates a reduction in its contractile potential [2]. LVNC is estimated to occur in 1:5000 adults [1]. Clinical symptoms can include heart failure, arrhythmia, and thromboembolism [4].
LVNC has not been widely reported in pregnancy, but outcomes described have been variable, often associated with heart failure [2]. This case examines a patient with known MYH7 genetic mutation and LVNC who had a pregnancy complicated by fetal Ebstein anomaly and LVNC seen on prenatal ultrasound examination. Mutation in MYH7 gene was later identified in the baby postnatally via genetic testing from cord blood. Following a vigilant multi-disciplinary surveillance strategy, the antenatal course and delivery were relatively uncomplicated. Both mother and baby have remained stable with their cardiac function to date. This case emphasizes the importance of prenatal diagnosis of LVNC and the need for genetic testing for the MYH7 gene mutation.
Our patient is a 43-year-old G5P1212 non-Hispanic black female with a medical history complicated by thoracic scoliosis status-post spinal fusion at age 15 years and history of open patch closure via sternotomy of a ventricular septal defect (VSD) at 9 months of age. Her history is interesting in that she had been followed by cardiology after repair of her VSD but had not been diagnosed with LVNC. She was subsequently diagnosed with LVNC at 34 years after delivery of a 30-week intrauterine fetal demise with autopsy revealing an Ebstein anomaly, hydrops, and genetic testing positive for the MYH7 gene mutation. At the time of our patient's initial diagnosis, she was non-pregnant, asymptomatic and underwent routine echocardiographic follow-up revealing a mildly diminished left ventricular ejection fraction (LVEF) of 47%, and diagnosis of LVNC by a measurement of the ratio of non-compacted to compacted myocardium revealing a ratio of 4.25:1 (normal value < 2:1) [3]. This diagnosis was confirmed by cardiac MRI. She ultimately underwent genetic testing identifying the MYH7 mutation - considered pathogenic for LVNC in this patient's family. Following genetic confirmation, the patient was encouraged to initiate a medication regimen including an ACE inhibitor, beta blocker, low dose aspirin, and diuretic, however the patient's compliance with these medications varied over time. Periodic echocardiograms and cardiac MRIs demonstrated stability, and she remained asymptomatic for 10 years from the time of her initial diagnosis until her most recent pregnancy.
Her obstetric and family histories are notable for an autosomal dominant pattern of inheritance for her MYH7 mutation. A spontaneous first trimester loss complicated her G1 where genetic testing was not performed. She subsequently experienced an uncomplicated term spontaneous vaginal delivery in her G2 affected by the MYH7 gene mutation, and she then experienced spontaneous preterm premature rupture of membranes with delivery at 34 weeks' gestation in her G3 where the newborn was not affected by the gene mutation. In her G4 pregnancy, the fetus demonstrated an Ebstein anomaly and ultimately suffered in utero demise followed by preterm vaginal delivery at 30 weeks. It was not documented that this fetus was tested for MYH7 gene mutation. The patient has 2 full sisters and a half-brother. One sister has LVNC and has not undergone genetic testing, but this sister also has a daughter with Ebstein anomaly.
In the antecedent pregnancy, the patient presented for prenatal care at 20 weeks' gestation and was managed through our multidisciplinary cardio-obstetric program. Aside from diet-controlled gestational diabetes, her pregnancy was uncomplicated. A maternal echocardiogram performed early in gestation revealed a left ventricular ejection fraction (LVEF) of 50-55% with thickening of the septum. This was followed by cardiac MRI which revealed a right ventricular ejection fraction of 47% (49-83%), LVEF of 65%, and a non-compaction to compaction ratio of > 2.2 in the noncompacted left ventricle (Figure 1). She was started on 162 mg Aspirin to decrease risk of micro emboli formation, but otherwise no significant changes were made to her prenatal care.
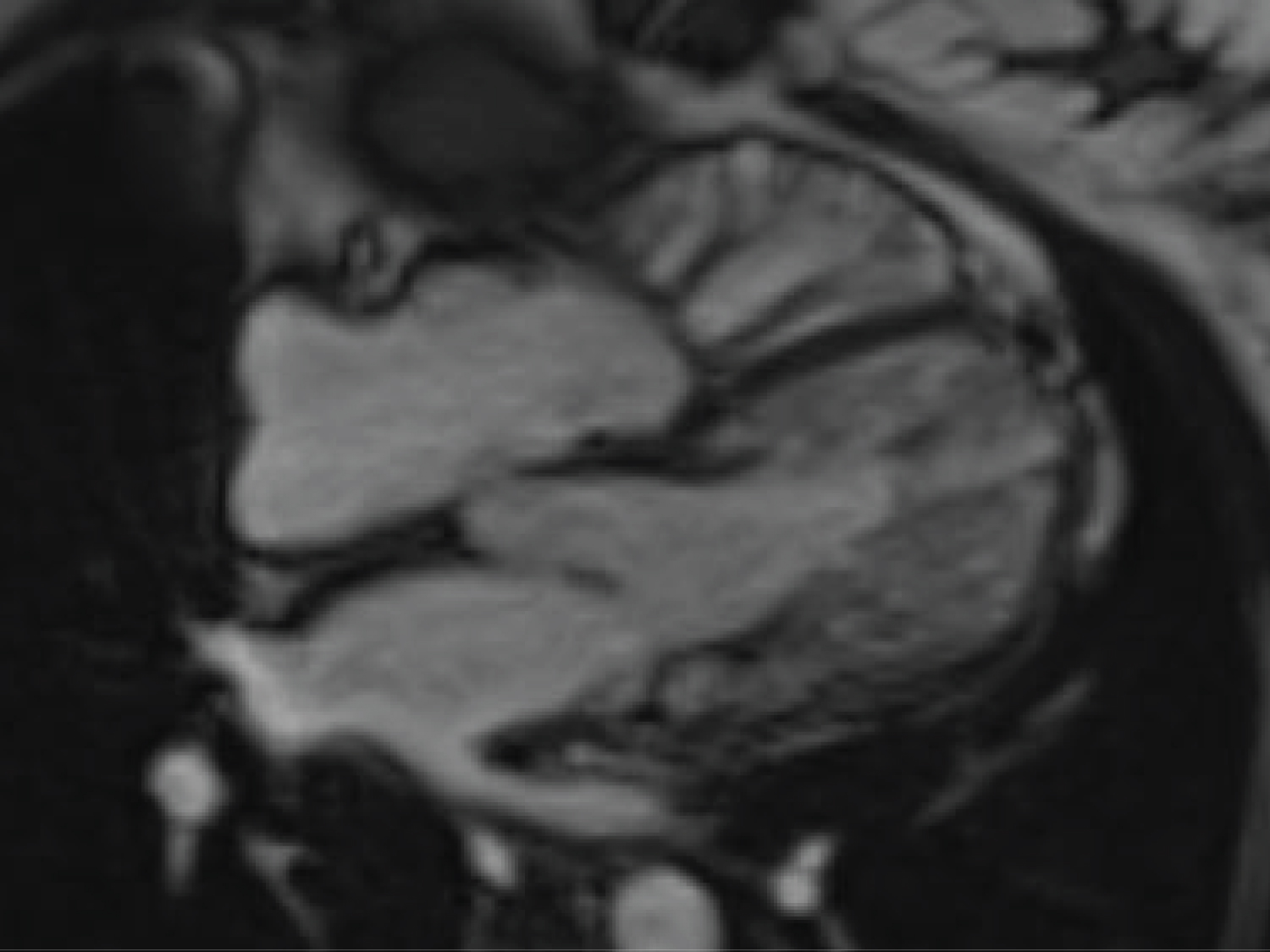 Figure 1: Maternal cardiac MRI 4-chamber view, note thickened left ventricle, noncompacted left ventricle.
View Figure 1
Figure 1: Maternal cardiac MRI 4-chamber view, note thickened left ventricle, noncompacted left ventricle.
View Figure 1
The fetal anatomic survey raised concern for a single umbilical artery, ventricular septal defect, and possible trabeculations in both cardiac ventricles (Figure 2). A dedicated fetal echocardiogram subsequently demonstrated a dilated right ventricle with moderately depressed systolic function, significant hypertrophy of the ventricular apex with deep trabeculations, mildly depressed left ventricular systolic function with ill-defined endocardial borders, deep apical trabeculations, and mild apical displacement of the tricuspid septal valve leaflet and subsequent tricuspid regurgitation - concerning for fetal LVNC and Ebstein anomaly (Figure 3 and Figure 4). No ventricular septal defect was identified. Overall, the constellation of findings strongly suggested inheritance of the MYH7 variant from the mother, but the patient declined prenatal genetic diagnosis.
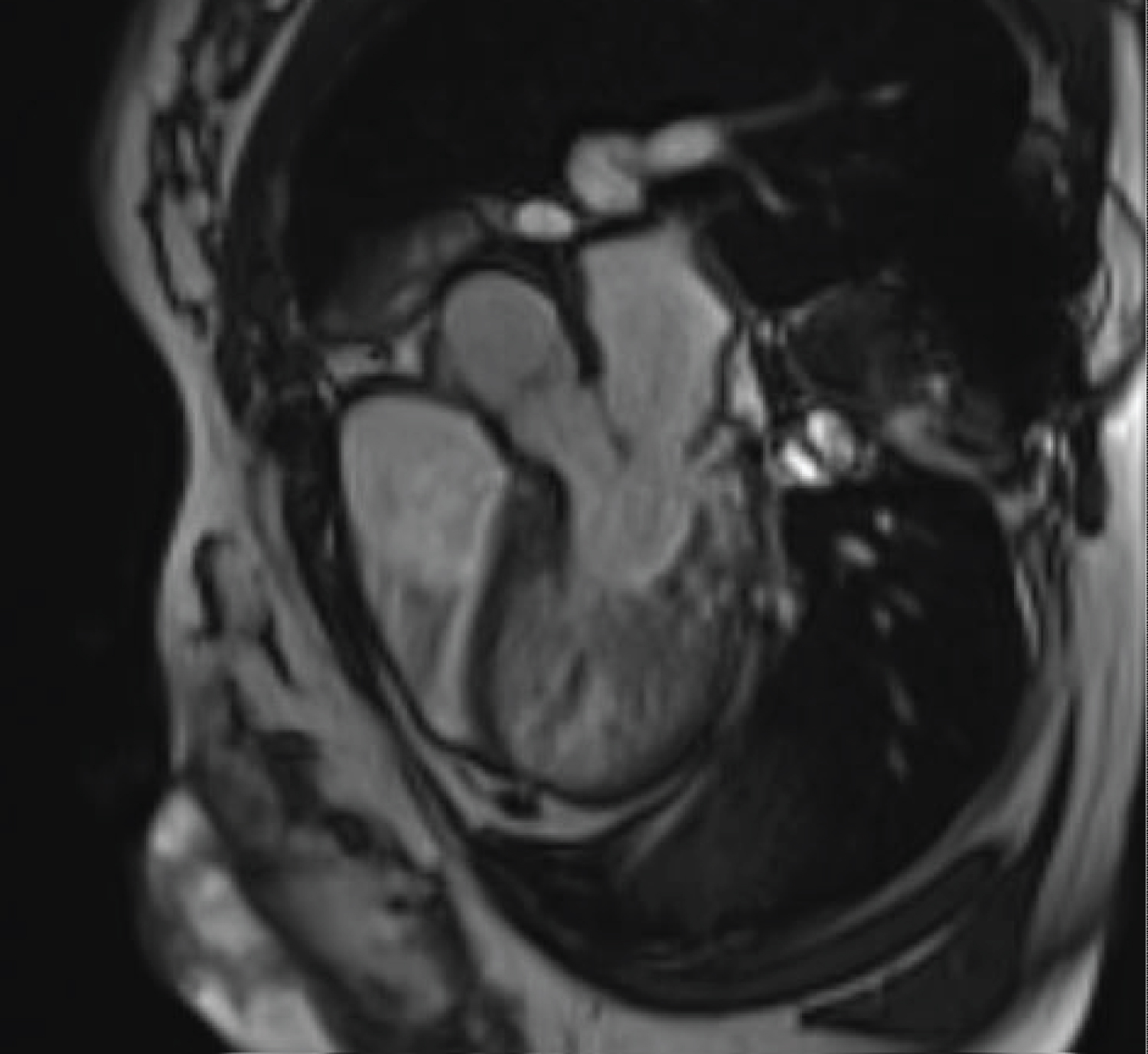 Figure 2: Maternal cardiac MRI demonstrating thickened left ventricle in LV outflow view.
View Figure 2
Figure 2: Maternal cardiac MRI demonstrating thickened left ventricle in LV outflow view.
View Figure 2
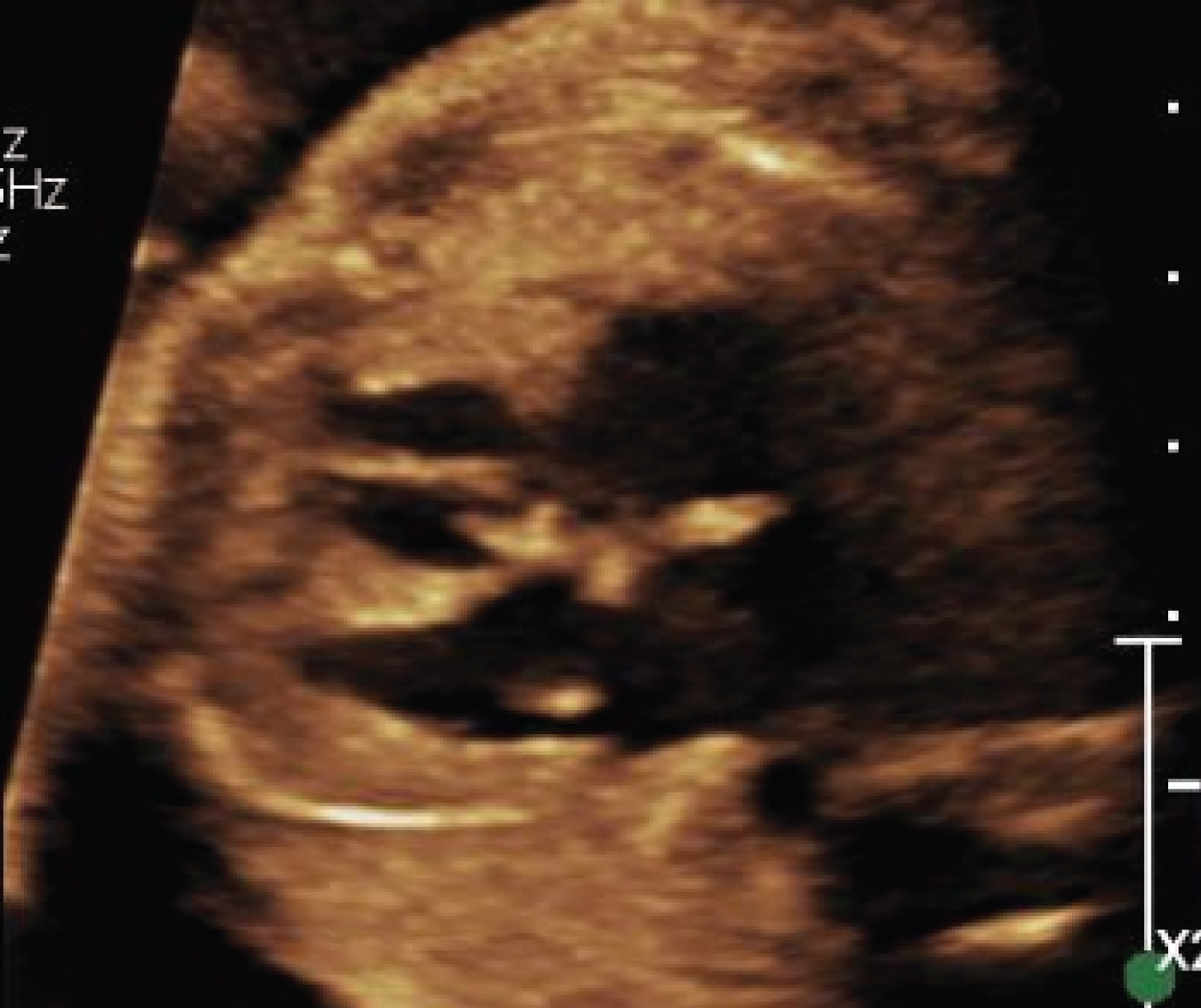 Figure 3: Fetal ultrasound with 4-chambered view. Initially with concern for possible ventricular septal defect and possible trabeculations in both cardiac ventricles; significant hypertrophy of the ventricular apex with deep trabeculations, mildly depressed left ventricular systolic function with ill-defined endocardial borders, deep apical trabeculations, and mild apical displacement of the tricuspid septal valve leaflet and subsequent tricuspid regurgitation - concerning for fetal LVNC and Ebstein anomaly.
View Figure 3
Figure 3: Fetal ultrasound with 4-chambered view. Initially with concern for possible ventricular septal defect and possible trabeculations in both cardiac ventricles; significant hypertrophy of the ventricular apex with deep trabeculations, mildly depressed left ventricular systolic function with ill-defined endocardial borders, deep apical trabeculations, and mild apical displacement of the tricuspid septal valve leaflet and subsequent tricuspid regurgitation - concerning for fetal LVNC and Ebstein anomaly.
View Figure 3
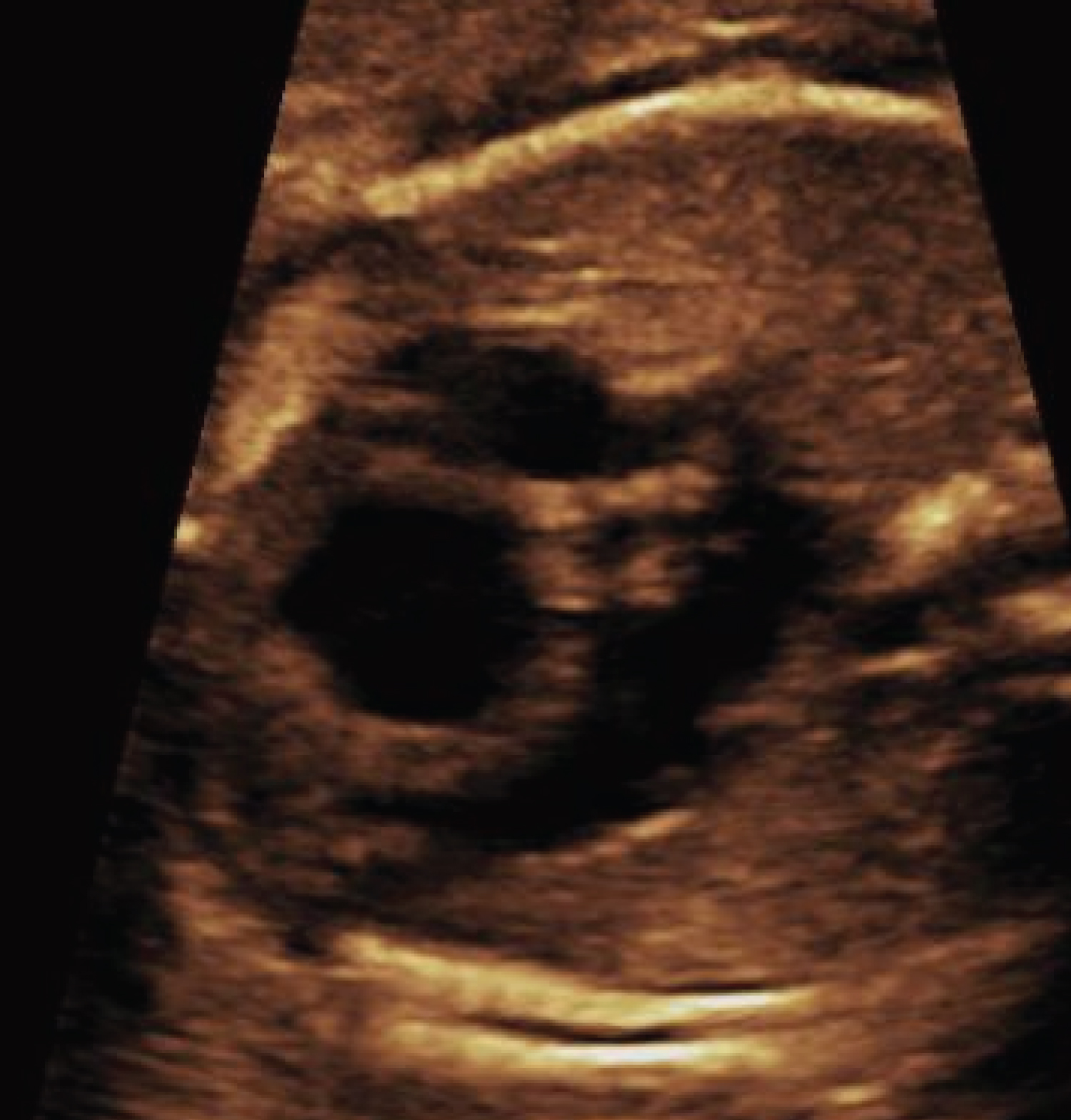 Figure 4: Fetal ultrasound demonstrating a dilated right ventricle with significant hypertrophy.
View Figure 4
Figure 4: Fetal ultrasound demonstrating a dilated right ventricle with significant hypertrophy.
View Figure 4
The patient underwent successful induction of labor at 37 weeks 5 days gestation resulting in normal spontaneous vaginal delivery of a female infant weighing 2744 grams with Apgar scores of 9 and 9 who was evaluated briefly in the special care nursery. Telemetry during labor and the postpartum period revealed no arrythmias. Daily metoprolol succinate 25 mg was initiated postpartum day 1. Both mother and baby were discharged home on post-partum day 2, and their postpartum/newborn courses were uneventful without complication. The infant's cord blood was sent for genetic testing demonstrating positivity for the MYH7 gene mutation.
LVNC is a rare condition in pregnancy but has shown an increase in prevalence over recent years. With the increase in diagnosis, there are likely to be more women of childbearing age contemplating and undergoing pregnancy with this condition, necessitating guidance for pregnancy counseling and management. In this case, we described the obstetrical course of a patient with known LVNC and MYH7 mutation in addition to the prenatal diagnosis of fetal LVNC which was later confirmed to have the MYH7 mutation as well. Delivery occurred at term and was uncomplicated with successful outcomes for both mother and baby.
Evidence suggests that pregnancy itself may possibly contribute to reversible de novo left ventricular trabeculation formation secondary to the increased preload associated with the volume expansion of pregnancy [5]. Gati, et al. evaluated 102 low-risk women throughout pregnancy and the postpartum period with serial echocardiography to assess changes among otherwise normal hearts [5]. New trabeculations appeared in 26 (25.4%), and these were noted to resolve during the postpartum period among the majority. However, 7 of the 26 demonstrated persistent trabeculations which remained stable without demonstrable evidence of cardiac dysfunction on subsequent serial echocardiography [5,6]. These findings emphasize the importance of distinguishing non-pathologic, reversible LVNC from pathologic LVNC as it drastically changes pregnancy management and counseling. Factors including ventricular dysfunction, familial or history of congenital heart defects, or unexplained cerebrovascular accident should prompt genetic evaluation as exemplified by this case.
Serious complications can be associated with pathologic LVNC which is why distinguishing between the two is vital. LVNC can lead to heart failure, arrhythmias, thromboembolism and cerebrovascular accidents. While many patients remain asymptomatic, these risks are especially pertinent in pregnancy given the normal physiological changes observed - a 50% increase in plasma volume, stroke volume, and cardiac output; which could lead to deterioration in cardiac function even in patients that were previously stable [5].
There is little published on LVNC management guidelines in pregnancy. Consensus remains that all patients with maternal cardiomyopathy should be managed with vigilant cardiac and obstetric surveillance - often best achieved through participation by a multi-disciplinary team of obstetricians and cardiologists [7]. Patient reported symptoms should prompt investigation and cardiac function should be assessed routinely throughout pregnancy. Given the paucity of data to guide anticoagulation management, our approach includes initiation of low-dose (81-162 mg) aspirin and reserve prophylactic anticoagulation for depressed ejection fraction (< 35%) or atrial fibrillation which is well supported by the literature [1,8]. Antenatal testing, serial obstetric ultrasound imaging and fetal echocardiograms should be included as part of the routine management for patients affected by LVNC. While in certain circumstances cesarean section may be required due to cardiac considerations, this case supports the safety of induction of labor with unassisted vaginal delivery for women with maintained cardiac function with cesarean delivery reserved for normal obstetrical indications. For those undergoing labor, continuous cardiac monitoring should be implemented with consideration for early neuraxial anesthesia to mitigate arrhythmia risk by selectively blocking sympathetic input [9].
This case is of particular importance due to the implicated genetic mutation. MYH7 is a sarcomere gene variant that demonstrates an autosomal dominant inheritance pattern which has been linked to an increased risk of fetal LVNC [8,10]. This underscores not only the importance of genetic counseling prior to conception for patients with a personal or family history of cardiomyopathy, but also the need for increased fetal surveillance during affected pregnancies. Importantly, findings on fetal echocardiography can differ from that of adults, with a larger proportion involving the right ventricle, perhaps related to differences in fetal circulation [11]. Decreased LVEF and higher N/C ratio have been demonstrated as independent risk factors for death in patients with fetal onset disease [12].
Patients affected by LVNC in pregnancy can have a wide range of clinical outcomes, and decisions regarding timing and mode of delivery should be based upon individual symptoms and cardiac function, rather than the diagnosis of LVNC alone [10,13,14]. This case supports the growing body of literature that maternal LVNC can be safely managed in pregnancy and that prenatal diagnosis of an affected fetus is possible even when genetic analysis is deferred until after delivery.
The authors have no conflicts of interest to disclose.