Obesity has become a worldwide epidemic disease. It is a complex disease involving an excessive amount of body fat. Obesity isn't just a cosmetic concern. It's a medical problem that increases the risk of other diseases and health problems, such as heart disease, diabetes, high blood pressure and certain cancer. Besides, it has been pointed out as a vital cause of kidney diseases. Due to its close association with diabetes and hypertension, excess weight and obesity are important risk factors for chronic kidney disease (CKD). Early dietary intervention should be considered in the management of overweight and obese chronic kidney disease (CKD) patients to reduce weight, and where necessary bariatric surgery. Further studies are required to better understand the association between obesity and kidney disease.
Obesity is a multifactorial chronic disease stemmed from long-term positive energy balance which leads to excess adiposity and subsequent functional impairment, structural anomalies and physiological disorders. Generally people are considered obese when their body mass index (BMI) which obtained by dividing a person's weight by the square of the person's height is over 30 kg/m2. Obesity is correlated with various diseases and conditions, especially type 2 diabetes, cardiovascular diseases, chronic kidney disease, and obstructive sleep apnea. It increases the risk for other chronic medical conditions and has been associated with premature death [1].
The global prevalence of obesity grew from less than 1% to 11% among men, and 6% to 15% among women [2]. In Egypt, the prevalence of obesity has increased in adults to reach about 40% according to 100 million health survey (2019) compared to the 36% estimate of 2017 STEP wise survey. Obesity is more prevalent in Egyptian males compared to females (about 30% compared to 50% respectively) [3].
Several factors may play a vital role in gaining excess weight. These include diet, lack of exercise, environmental factors, genetics and hormonal changes.
People gain weight when they eat more calories than they burn through activity. This imbalance is the greatest contributor to weight gain [4].
Lifestyle plays a significant role in obesity. Worldwide there has been a large shift towards less physically demanding work besides, insufficient exercise and increasing use of mechanized transportation in presence of Food advertising encourages people to buy unhealthy foods, such as high-fat snacks and sugary drinks [5].
In some people, obesity can be traced to a medical cause, such as Prader-Willi syndrome, Cushing syndrome and other conditions. Medical problems, such as arthritis, also can lead to decreased activity, which may result in weight gain. Some medications can lead to weight gain if you don't compensate through diet or activity. These medications include some antidepressants, anti-seizure medications, diabetes medications, antipsychotic medications, steroids and beta blockers [6].
The genes that inherit from the parents may affect the amount of body fat store, and where that fat is distributed. Genetics may also play a role in how efficiently the body converts food into energy, how it regulates appetite and how it burns calories during exercise. Obesity tends to run in families. That's not just because of the genes they share. Family members also tend to share similar eating and activity habits [7].
Obesity can be associated with several endocrine alterations arising as a result of changes in the hypothalamic-pituitary hormones axes. These include hypothyroidism, Cushing’s disease, hypogonadism and growth hormone deficiency. The hormones growth hormone, leptin, oestrogens, androgens and insulin influence our appetite, metabolism and body fat distribution. People who are obese have hormone levels that encourage the accumulation of body fat [8] (Figure 1).
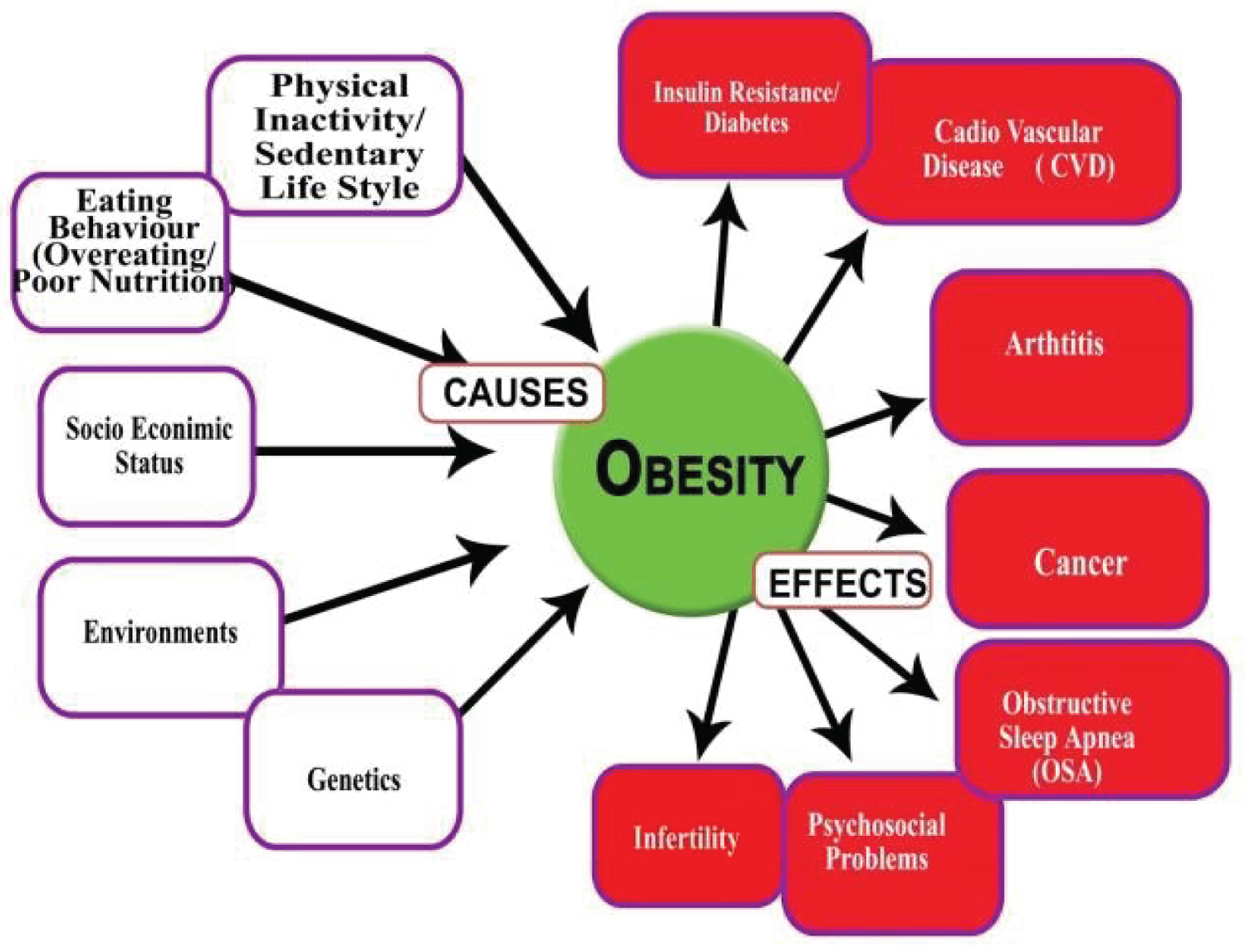 Figure 1: The main causes and some of consequences of obesity [9].
View Figure 1
Figure 1: The main causes and some of consequences of obesity [9].
View Figure 1
Increased prevalence of obesity was followed by increased prevalence of cardiovascular disease, hypertension, and diabetes. The adverse effects of peripheral insulin resistance and hypertension coupled with dyslipidemia and systemic inflammation may trigger the development of chronic kidney disease (CKD) [10]. The deleterious effects of obesity on renal function may occur indirectly via diabetes mellitus and/or hypertension - or directly by the production of adipokines, which trigger the onset of oxidative stress, inflammation, aldosterone system, activation of the renin-angiotensin, abnormal lipid metabolism, increased insulin production and insulin resistance. These factors result in ectopic lipid accumulation in renal tissue, leading to functional and structural impairment of podocytes, mesangial cells, and the proximal tubule, culminating with albuminuria, glomerular hypertension, increased glomerular permeability, hyperfiltration, and glomerulomegaly even focal segmental glomerulosclerosis (FSGS) in some cases [11].
Human obesity is a complex disorder, with many factors playing a part; the pathophysiology of adipokines is not as simple as it seems to be in rodent models of obesity. Obesity is associated with hemodynamic, structural and histopathological alterations in the kidney, as well as metabolic and biochemical alterations that predispose to kidney disease, even when renal function is normal in the conventional tests [12]. It is currently known that adipose tissue is not only a fat reservoir, but a dynamic tissue involved in the production of "adipokines", such as leptin, adiponectin, monocyte chemoattractant protein-1, transforming growth factor-β, tumor necrosis factor-α, and angiotensin-II [13].
A series of events is triggered by obesity, including insulin resistance, glucose intolerance, hyperlipidemia, atherosclerosis and hypertension, all of which are associated with increased cardiovascular risk. The association between CKD and dyslipidemia has also been described, but its causes are still unknown. Insulin action resistance, present in CKD, reduces the activity of lipoprotein lipase, which may be implicated in the pathophysiology of dyslipidemia in CKD [14].
Obesity leads to increased renal tubular sodium reabsorption, impairing pressure natriuresis and causing volume expansion due to the activation of the sympathetic nervous system (SNS) and the renin-angiotensin-aldosterone system (RAAS). Compression also occurs in the kidneys, especially when visceral obesity is presented [15].
The increase in sodium reabsorption and consequent extracellular volume expansion is a central event in the development of SAH in obesity. Some evidence suggests that an increase in sodium reabsorption occurs in some segments in addition to the proximal tubule, possibly in the loop of Henle. Also, there is an increase in renal blood flow, glomerular filtration rate (GFR) and filtration fraction [16].
Glomerular hyper filtration, associated with increased blood pressure and other metabolic alterations such as insulin resistance and DM, finally result in kidney damage and reduced GFR. The sympathetic nervous system (SNS) activation also contributes to obesity-related hypertension. There is evidence that renal denervation reduces sodium retention and hypertension in obesity, suggesting that SNS activation induced by obesity increases blood pressure mainly due to the sodium retention stimulus, rather than vasoconstriction [15].
Adipose tissue accumulation, especially in visceral adiposity, causes kidney compression, and a consequent increase in intrarenal pressure. The excess of retroperitoneal adipose tissue involves the kidneys and penetrates the renal hilum up to the medulla, causing compression of the renal medulla and increased hydrostatic pressure of the renal interstitial fluid. Excess visceral fat also increases the intra-abdominal pressure, causing further renal compression. The physical compression of the kidney causes increased extracellular matrix formation in the renal medulla. As the kidneys are surrounded by a capsule, which has low compliance, extracellular matrix accumulation can further exacerbate intrarenal compression and increase the hydrostatic pressure of the interstitial fluid. This increase in intrarenal pressure, in turn, compresses the loop of Henle and peritubular capillaries (vasa recta), which reduces the flow of fluids through the renal tubules leading to sodium reabsorption by them [12].
Obesity is also associated with inflammation, as an increase in the production of inflammatory cytokines such as tumor necrosis factor-α, interleukin-6 and C-reactive protein can be observed, being called "adipokines" by some authors because of their production by adipocytes. Inflammation itself is a risk factor for renal function loss. Renal fibrosis (interstitial and glomerular), in addition to the irreversible accumulation of extracellular matrix in kidney tissue, is associated with inflammation, processes that may be associated with the "adipokines" [15].
There is also evidence that obesity itself increases albumin excretion, which progressively increases with obesity severity and, in rare cases, can lead to nephrotic syndrome [16]. Obesity has also shown to be an important risk factor for renal disease progression in patients with glomerulopathies, such as in IgA nephropathy [17].
Focal and segmental glomerulosclerosis (FSGS) is the type of glomerulonephritis most often associated with obesity. FSGS associated with obesity typically presents with nephrotic syndrome and progressive renal function loss. The morphological findings include glomerulomegaly, predominance of perihilar variant and mild podocyte fusion. Weight loss, either by dieting or through bariatric surgery, leads to proteinuria reduction [18].
The association between obesity and nephrolithiasis has been observed in some studies, mainly due to increased serum uric acid levels in obese individuals [19]. Although obesity represents a major risk factor for the development of cardiovascular disease, some studies have shown that obesity is a protective factor in individuals with end-stage CKD (undergoing dialysis), perhaps because malnutrition is associated with higher mortality when compared to obesity. However, even in patients undergoing dialysis, visceral obesity is associated with increased risk of coronary calcification and adverse cardiovascular events [20] (Figure 2).
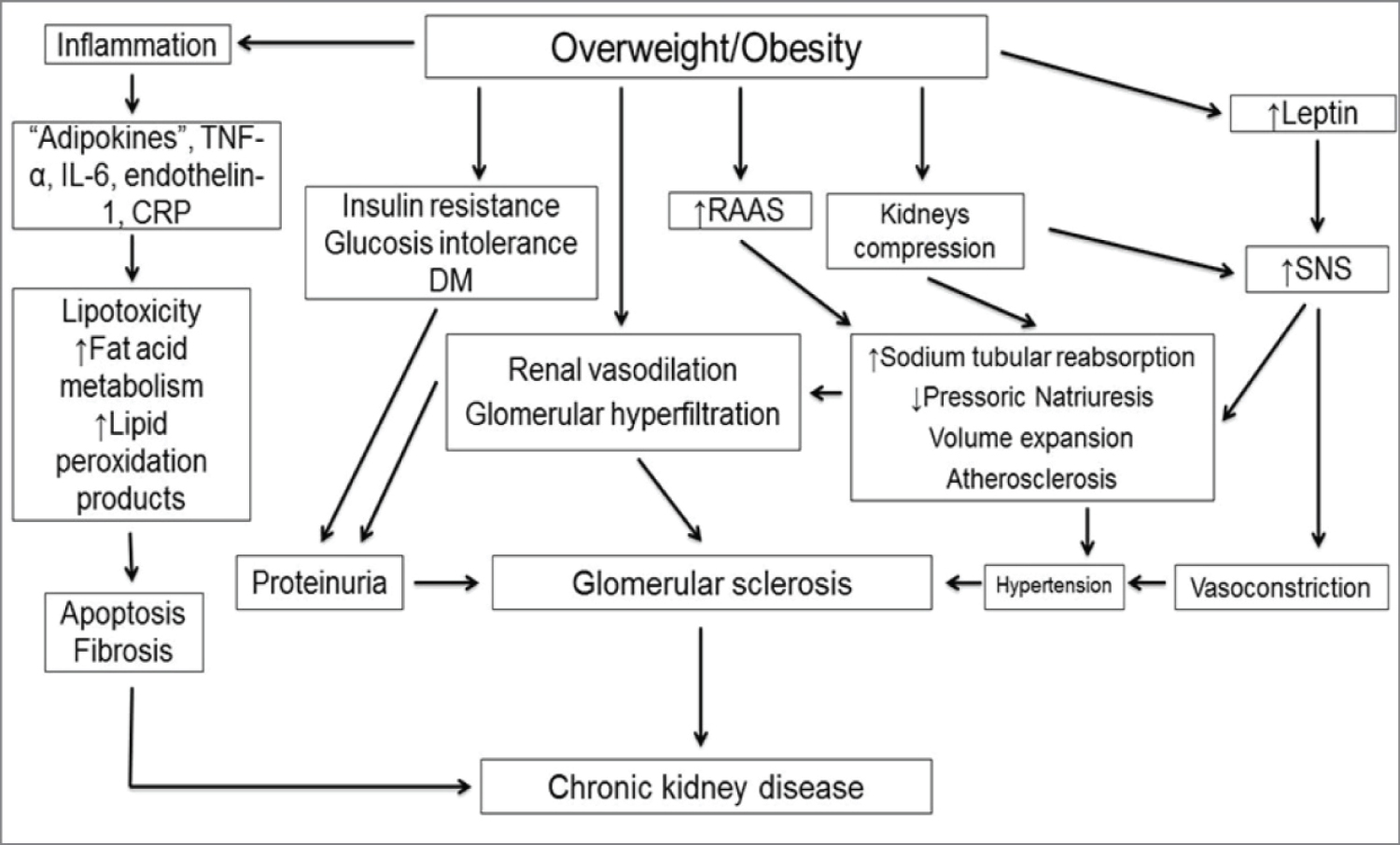 Figure 2: Pathophysiology of the association between obesity and kidney disease [20].
View Figure 2
Figure 2: Pathophysiology of the association between obesity and kidney disease [20].
View Figure 2
None.
The author has nothing to disclose.