Misbranded and counterfeit dietary supplements have been an issue on which the US Food and Drug Administration has been vigilantly regulating. The ubiquity of online-shoppable weight-loss supplements and their unrestricted consumption by people with obesity are serious matters of concern. Fucoxanthin, a brown-seaweed-extracted carotenoid has exhibited anti-obesity property in some clinical trials through its ability to over express uncoupling protein (UCP1) in the white adipose tissue, which leads to fat burning. However, since the clinical trials apply pure fucoxanthin instead of fucoxanthin-bearing dietary supplements, a critical analysis is warranted to quantify fucoxanthin in brown seaweeds to validate their rationale in weight loss mechanism. This study examined ten randomly chosen online-sourced brands of fucoxanthin-containing dietary supplements and analyse the content using High-Performance Liquid Chromatography (HPLC) equipped with UV-Vis and photodiode array detector. Our exploration revealed that, of these 10 products, 3 (30%) did not have any detectable quantity of fucoxanthin, 5 (50%) contained a only a trace amount of it, ranging from 0.001-0.01 mg per capsule, and only 2 (20%) products contained 0.4 mg or 2 mg of fucoxanthin, meeting their label claim. This worrying finding may be interpreted that the existing ease-of-access and pervasive nature of online-sourced dietary supplements require more stringent regulatory screening.
Fucoxanthin, Weight loss, Dietary supplements, Misbranding, Quality assurance
UCP1: Uncoupling Protein; HPLC: High-Performance Liquid Chromatography; NIH: National Institutes of Health; BMI: Body Mass Index; DSHEA: Dietary Supplement of Health and Education Act of 1994; cGMP: Current Good Manufacturing Practice; DSF: Dietary Supplement Fraud; LOD: Limit of Detection
Obesity continues to contribute adversely to our health, in fact, obesity continues to cause, influence and worsens cardiovascular diseases such as hypertension, stroke and myocardial infarction, obesity also has a major effect on the endocrinology system leading to the development of Type 2 Diabetes including the secretions of inflammatory cytokines which has been linked to Cancers of (Endometrium, Breast, Colon, Kidney, Gallbladder, and Liver) - all of which are life-threatening and costly. The Epidemiology of obesity shows a consistent growth pattern of increasing body weight in the United States. According to a recent survey, 93.3 million US adults (39.8%) and 17% of US teenagers are classified obese or overweight in the year 2015-2016 [1]. In addition to its serious health challenges. Its financial burdens balloons yearly without limitations. The estimated annual health care costs of related illness stand at a staggering $190.2 billion which equals nearly 21% of annual medical spending in the United States. The National Institutes of Health (NIH) classifies an individual to be obese when their measured or calculated BMI (Body Mass Index) equals or exceeds 30 Kg/m2. Several organizations and Experts have proposed different tools for classifying obesity based on the limitations of NIH guidelines such as differences in body composition, muscle mass, demographics, and other genetic contributors [2]. Obesity also tends to negatively impact individuals psychologically thereby leading to poorer mental outcomes, loss of productivity, low self-esteem, bullying, and stigmatization [3]. Obesity has been heavily studied and debated over the past decade as scientists continue to wobble about the effective long-term solutions to control this epidemic. Conventionally, consuming fewer calories, increased exercise continues to determine our outcomes. The overall sustainability remains a major challenge of all individuals as recent evidence suggests a significant number of people who lose weight actually eventually gain back all the weight back after 3 years [4].
Different strategies have been employed by individuals to reduce body weight, most of which are behavioral to control diet and physical activity. Since diet and physical exercise is usually insufficient, use of pharmacotherapy and surgery remains an option [5,6]. Most individuals are usually hesitant in using prescription due to the known side effects and general in availability. NIH has made recommendations regarding the use of prescriptions drugs and supplements in aiding weight loss especially in individuals with a BMI>/= 30 or for patients with a BMI equals or greater than 25 and 29.9 or High-risk waist circumference, with two or more risk factors [7]. Weight loss surgery is an option for patients with extreme obesity/ clinically severe obesity BMI >/= 40 OR a BMI >/= 35 and serious comorbid conditions. However, there are more risks associated with surgery, it is usually more expensive, and the resources may not be available [8,9]. Moreover, since most prescription FDA approved medications carries serious adverse effects and regularly requires physician oversight and several quantity restrictions and classification as DEA controlled medication, most people rely heavily on the use of inexpensive over the counter supplements to assist in losing weight [10].
Fucoxanthin is one of the most abundant carotenoids which is found majorly in marine environments, especially as a composition of seaweeds, microalgae, and diatoms. When viewed under a microscope, it appears as an orange colored pigment juxtaposed with other orange-colored cells, such as chlorophyll and many subtypes of Carotenes found predominantly in sea organisms [11]. Evidence suggests acceptable beneficial effects in increasing metabolism, suppressing ghrelin, increasing Leptin and modulating the digestive and immune system. The mechanism in which it helps prevent weight gain and accelerate weight loss is not widely understood, but researchers suggest it impacts the hepatic system which helps in controlling cholesterol, lipid synthesis and fatty acid degradation [11,12]. Food supplements are frequently used in weight loss for one of two reasons: a) providing nutrients that may be lacking in calorie-restricted diets, and b) potential benefits in enhancing weight loss. There are many botanical products that claim to enhance weight reduction, although the evidence of their efficacy is often inconclusive [13].
Dietary supplements are products that are made to supplement, replace, augment or introduce a dietary ingredient that individuals are deficient, lack or needs. Although most dietary products are available to use orally, the definitions or classification of these products is generally wide, vague or too complex for a consensus agreement. Prescription drugs undergo extensive clinical and laboratory trials involving an in-depth understanding of their physicochemical, pharmacodynamic, and pharmacokinetic profile and must be approved by the Food Drug Administration (FDA) before they can be marketed. Dietary supplements do not require premarket approval by the FDA. Since dietary products do not undergo extensive clinical trials as prescriptions drug is often not validated in terms of overall safety or efficacy profile, the (DSHEA) Dietary supplement of health and education of 1994 strives to prohibit manufacturers from making unsubstantiated claims, distribution of unsafe or counterfeit molecular entities to the public. DSHEA mandates the manufacturer to ensure the safety and labelling of their products before they are marketed. The manufacturer does not have to provide that evidence to the FDA. However, they must establish and follow current good manufacturing practices to ensure the quality of the dietary supplements [14,15].
Quality control testing is essential for numerous reasons. It provides a competitive advantage along with ensuring the safety of its consumers and providing a company with credibility. Every member of a company is responsible for assuring quality starting at the top management to provide training and education, and including all employees to follow quality control procedures [16]. Dietary supplements today due to wilful economic gain have resulted to the manufacturing of products which are misbranded and sometimes adulterated [17]. Most supplements companies advertise a certain active ingredient or compound that has been associated with significant positive health benefits but that agent may not be present in the final product. Sometimes sufficient quantity is not provided, but the label carries entirely different specified quantity [18]. Adverse health events resulting from the dietary supplement fraud represents a significant public health threat. Consequences are increasing with the growing consumption of these misbranded and sometimes adulterated supplements [19,20]. Americans spent about $36.7 billion dollars on dietary supplements (vitamins, minerals, amino acids, and herbs) [21]. However, the majority of this was spent on three major categories weight loss, bodybuilding, and sexual function. Weight loss due to the prevalence of obesity in the United States has led many people to choose a dietary approach to lose weight, as such is using different supplements as a meal replacement. However, due to the overwhelming proportion of misleading information regarding their use, many people find it difficult to choose the right supplements [22]. Many consumers attribute higher price supplements to mean they are more efficacious and of higher quality, which may not necessarily be true. Many companies make claims to consumers about the effectiveness of a product, which cannot be substantiated due to their lack of compliance with what they put in their product. The main objective of the study was to determine the content of Fucoxanthin in dietary supplements available in US market.
All solvents used in this study were HPLC grade and procured from commercial suppliers. Acetonitrile were purchased from EMD Millipore, MA, USA, and the purified water was obtained through the in-house water distillation system, model SZ-93A, manufactured by Huanyu, China. Fucoxanthin (CAS Number 3351-86-8) was purchased from Sigma Aldrich and the fucoxanthin supplements were purchased from online retailers in the USA.
The sample preparation and chromatographic methods used in this study were adopted and modified from GL Sciences [23]. In brief, the powder sample (equivalent to 2.0 mg of Fucoxanthin) was placed in 25 mL of Acetonitrile in a 50 mL volumetric flask and sonicated for 5 min. Then. 25 mL of diluent (the mixture of acetonitrile and purified water in the ratio of 1:1) was added to the preparation and again sonicated for 5 min. Then, the contents were allowed to cool at room temperature and adjusted the volume up to 50 mL using diluent (if necessary). The solution was then filtered through 0.45 μm membrane filter. After which, 1.0 mL of the filtered solution and 1.0 mL of the diluent was transferred to an Eppendorf tube and vortexed. Ten microliters of this solution were injected into the HPLC system to quantify the fucoxanthin content.
Liquid chromatography is one of the most powerful and most commonly used separation techniques, which has been using in food and pharmaceutical industry for its excellent sensitivity and specificity over Ultraviolet-visible spectroscopy technique. Generally, the content of organic compounds present in food and pharmaceutical products are determined by RP-HPLC integrated with UV-Vis Detector during quality testing. The chromatographic separation of the fucoxanthin was achieved by the Shimadzu prominence HPLC system; using a gradient elution mode on a Waters X-Select CSH C-18 column (4.6 mm × 150 mm, 5.0 µm particle size, Part no. 186005290). The HPLC system consisted of a quaternary pump (LC-20AD), a UV-Vis detector (SPD-20AC, having exceptional level of sensitivity and stability). Mobile phase consisted of 850 mL of Acetonitrile and 150 mL of purified water. The instrumental setup included the flow rate at 1.0 mL/min and the temperature of the column oven was at 30 ℃. The injection volume was set to 10 μL with a run time of 8 minutes. The effluent was detected at 447 nm based on the lambda max (Figure 1a) obtained using alliance 2695 separation module (Waters, MA, USA) connected to a Photodiode Array detector (Waters 2996, Waters, MA, USA) to get maximum detector response and sensitivity. A 1.0 mg/mL of fucoxanthin standard stock solution was prepared in Acetonitrile and then 5 µg/mL, 10 µg/mL, 15 µg/mL, 20 µg/mL and 25 µg/mL of fucoxanthin solutions were prepared from the stock solution using the diluting solvent to quantify fucoxanthin present in the test solution (fucoxanthin dietary supplements). All weights were measured using a pre-calibrated analytical balance Mettler Toledo PB303-S/FACT (Mettler Toledo, Switzerland).
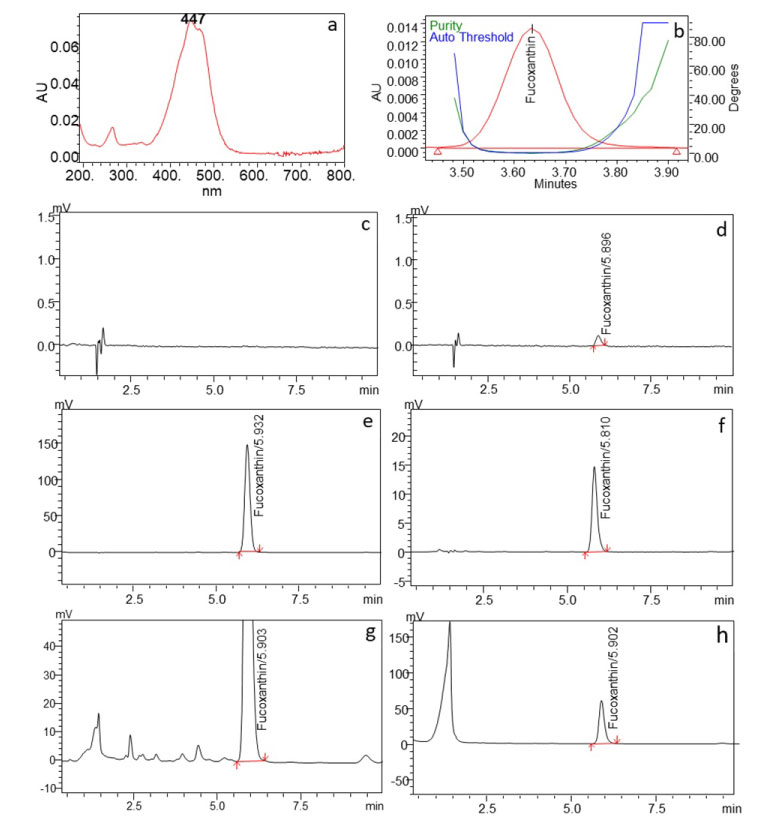 Figure 1: HPLC chromatograms obtained in assay test, a) UV-Vis Spectrum of Fucoxanthin; b) Peak purity plot; c) Diluting solvent; d) LOD solution; e) Identification and reference standards; f) Test sample spiked with reference standard; g) Test solution (DS02); h) Test solution (DS07). View Figure 1
Figure 1: HPLC chromatograms obtained in assay test, a) UV-Vis Spectrum of Fucoxanthin; b) Peak purity plot; c) Diluting solvent; d) LOD solution; e) Identification and reference standards; f) Test sample spiked with reference standard; g) Test solution (DS02); h) Test solution (DS07). View Figure 1
The reliability of the methodology used for the determination of fucoxanthin in the dietary supplements was confirmed by evaluating the specificity, sensitivity, linearity, limit of detection (LOD), reproducibility and solution stability [24,25]. Specificity is one of the most important parameter for an analytical method particularly for natural products because natural products contain a lot of unknown compounds. The specificity for the chromatographic method used in this study was confirmed by checking the blank interference, resolution between the adjacent peaks, and peak purity (spectral analysis) [26]. To evaluate the peak purity of the chromatogram the spectral analysis was achieved using vector analysis algorithms using Photodiode Array detector (Figure 1b). The purity angle (0.734) is found lower than the purity threshold (0.740) for the fucoxanthin peak, which indicates the peak is spectrally homogeneous and contains only one compound. HPLC chromatogram of the diluting solution clearly indicates that diluent and mobile phase has no interference in the studies because no peak is co-eluting at the retention time of 5.9 min (Figure 1c). Both of the test sample chromatograms (Figure 1g and Figure 1h) visibly indicate that peaks are well separated from each other, the resolution between the neighbouring peaks is more than 1.5. The responses for the proposed method was found to be linear (y = 84727370x + 25246, R² = 0.9995) from 5 µg/mL to 25 µg/mL (Figure 2). Detection limit of the methods was calculated based on the visual evolution as per ICH guidelines and FDA guidelines. LOD was found to be 0.67 µg/mL (Figure 1d). A known concentration of standard solution (100 µg) was spiked to the sample powder to evaluate the recovery and impact of the filter during filtration process. The recovery was found to be 102.3% and there was no interference of sonication and filtration was observed (Figure 1f). The solution was also found to be stable at room temperature for more than 48 hours.
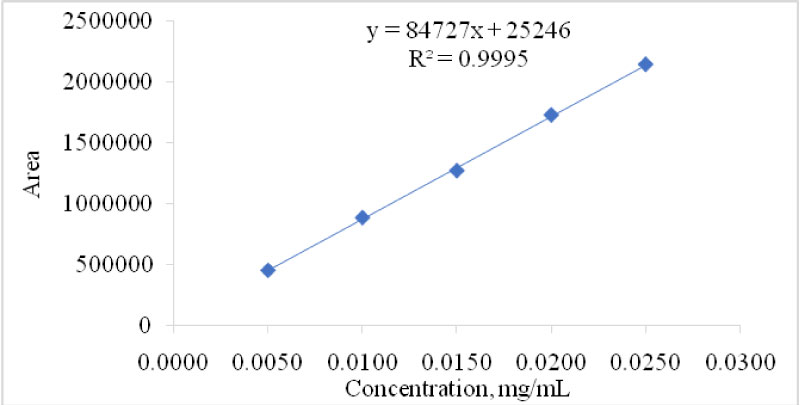 Figure 2: Linear curve (0.005 mg/mL to 0.025 mg/mL of Fucoxanthin). View Figure 2
Figure 2: Linear curve (0.005 mg/mL to 0.025 mg/mL of Fucoxanthin). View Figure 2
The brown seaweed dietary supplements purchased from the online retailers were analyzed immediately to quantify the content of fucoxanthin present in the products. Table 1 represents the testing results of the ten products. All the products were analyzed twice at different time points. Most of the products failed to meet their specifications as per their product label claim. The Results showed that 3 out of 10 products (DS05, DS06 & DS08) did not have any Fucoxanthin and 5 out of 10 products (DS01, DS03, DS04, DS09 & DS10) have trace amounts of Fucoxanthin ranging from 0.001 - 0.01 mg per capsule and only 2 out of 10 products (DS02 & DS07) contains 0.4 mg and 2 mg of Fucoxanthin which conformed with the amount stated on their supplement facts.
Table 1: Content of Fucoxanthin in dietary supplements. View Table 1
In general, the colour of carotenoids plant pigments is deeply yellow or orange, because they absorb wavelengths ranging from 400-550 nanometres. This is what gives brown seaweed raw material its characteristic yellow or orange colour. We have analysed some Fucoxanthin raw materials (Fucoxanthin 1%, 5%, 35%, & 50%, extracted from Brown Seaweed) to evaluate the root cause of the substandard products. Most of the fucoxanthin raw materials failed to meet their specifications analysed by HPLC. Other carotenoids plant pigments may present in the raw materials or the fucoxanthin raw materials may be contaminated with other chemicals (such as yellow/orange dye to the raw materials may make it appear to be compliant but contains little to none of the specified ingredient) that absorb at the same wavelength and give falsely UV absorbance. It is not a good practice to use UV-vis spectrophotometer to identify and quantify any ingredients in herbal products without purification. As, HPLC technique is more specific, accurate and precise than UV method, companies need to move away from using only UV-Spectrometry to determine the content of Fucoxanthin in brown seaweed raw material. HPLC analysis was able to show that there was very trace amount of Fucoxanthin in the products and moving forward the use of HPLC during quality testing should be the gold standard in the manufacturing of dietary supplements. In summary, using more sophisticated testing practices such as HPLC would further reduce the error in products being sent to the market.
The practice of using dietary supplements to help aid with weight loss and other ailments will continue to be a trend as these products are easily accessible and available without a prescription or consult from a healthcare provider. Manufacturers of these products are able to market them on a large scale to patients online, their local pharmacy, or other retailers. Patients trust that products being sold from a manufacturer meet current regulations as it is the responsibility of the company to self-regulate ensuring all products meet industry standards for cGMP before making them available. This self-regulation without direct oversight may create an environment where a company does not implement a proper system to verify the safety or quality of the product before being marketed to the public leading to potentially mislabeled and/or misbranded products ready for consumption.
In summary, our analysis revealed that a significant number of weight loss products containing Fucoxanthin in the market may be either misbranded or mislabelled and there may be a need for more oversight of dietary supplements by the FDA to protect consumers who are taking these products. Additional oversight would protect the everyday consumer from potentially harmful products or falling prey to false claims made by manufacturers.
The author(s) declare(s) that there is no conflict of interest regarding the publication of this paper.
The research was funded by the Internal Research Grant at Appalachian College of Pharmacy.
We have evaluated the Fucoxanthin content in popular weight loss supplements to support the following research project "Fucoxanthin for Diabetes Prevention and Control". All the authors are working on this project. Drs. Hossain, Rashid, Justice, and Abdelfattah are supervising this project and contributed to the final version of the manuscript. Drs. Burniston and Ahmed performed the analysis and helped in writing the manuscript. Drs. Wu, Kataye, and Sidhu discussed the results and contributed to the manuscript writing.