Biliopancreatic diversion (BPD) is considered the most effective procedure for the surgical treatment of morbid obesity [1]. First presented by Dr. Nicola Scopinaro in 1976, this operation proved safe and reproducible, with astonishing results regarding weight loss and weight maintenance for many years after the operation [2,3]. Possible late complications include anemia, stomal ulcer, bone demineralization, neurologic complications, and protein malnutrition.
A clear consequence of the operation is fat and bile acid malabsorption, and possibly carbohydrate malabsorption. As a result, it is reasonable to think that BPD patients may have altered microbiota in their colons, and that secondary to these changes, some changes in the immunologic functions in the colon can occur, rendering it more susceptible to insults. Here we report two unusual cases of unexplained colitis occurring in two female patients who underwent BPD.
Patient #1 is a 52-year-old woman who had undergone a Silastic Ring Vertical Gastroplasty (SRVG) for morbid obesity in 1980, with an initial Body Mass Index (BMI) of 64.6 Kg/m². She lost 33 Kg during the first year after the operation, but since then her weight was stable with no further reduction. In 1998 she underwent an open cholecystectomy. With a BMI of 52.6 Kg/m², she was referred to our department for a revisional bariatric operation. Laboratory test results taken preoperatively, at readmission, and at discharge, are shown in Table 1. She underwent an uneventful laparoscopic BPD. Some intraoperative measures are shown in Table 1. The early post-operative period was unremarkable. Some post-operative parameters are also shown in Table 1. Seven weeks after the operation she started to complain of vague right abdominal pain and watery diarrhea and was ultimately diagnosed with colitis of the hepatic flexure and proximal transverse colon, based on computerized tomography (CT) (Figure 1). Her laboratory tests were as shown in Table 1. Stool cultures were negative for Salmonella, Shigella, Campylobacter Jejuni, and common parasites routinely examined in our hospital. The stool was also negative for Clostridium Difficile toxins A and B. She was treated with intravenous empiric antibiotics, resulting in a quick clinical and laboratory improvement (Table 1). Six weeks after her discharge, a colonoscopy was performed, demonstrating a normal mucosa throughout the colon, with no signs of inflammation or any other pathology. Random biopsies taken from hepatic flexure mucosa were normal. Since that event, she reported no other attacks of abdominal pain, diarrhea, or any other complaints.
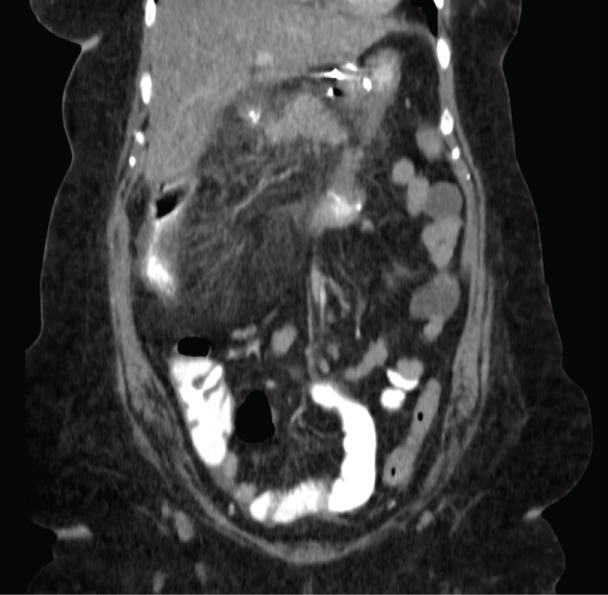 Figure 1: CT scan of patient #1, showing colitis of the hepatic flexure and proximal transverse colon. View Figure 1
Figure 1: CT scan of patient #1, showing colitis of the hepatic flexure and proximal transverse colon. View Figure 1
Table 1: Laboratory test results taken preoperatively, at readmission, and at discharge, as well as intraoperative measures, and post-operative parameters for the 2 patients. View Table 1
Patient #2 is a 43-year-old woman who underwent Silastic Ring Vertical Gastroplasty (SRVG) for morbid obesity in 1988, with an initial BMI of 62.2 Kg/m². She lost 61 Kg during the first few years following the operation, but since then she gradually gained weight. The patient was referred to our department for a revisional bariatric operation. The preoperative laboratory tests are shown in Table 1. In 2012 she underwent an uneventful laparoscopic BPD and cholecystectomy. Important intraoperative measures are shown in Table 1. The early post-operative period was unremarkable. Some post-operative parameters are also shown in Table 1. Eight weeks after the operation she was admitted to our department because of diffuse abdominal pain, diarrhea, vomiting, and fever up to 39 ℃, and was ultimately diagnosed with colitis extending from the cecum to the sigmoid colon based on a CT scan (Figure 2). Her laboratory tests were as shown in Table 1. As in patient #1, the stool cultures were negative. She was treated with intravenous antibiotics and a clear improvement was observed. One year later, a colonoscopy was performed, demonstrating a normal mucosa throughout the colon, and random biopsies taken from hepatic flexure mucosa were normal. Since that event, she reported no other attacks of abdominal pain, diarrhea, or any other complaints.
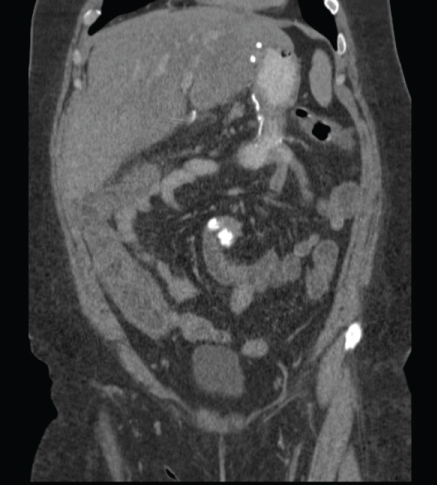 Figure 2: CT scan of patient #2, showing colitis extending from the cecum to the sigmoid colon. View Figure 2
Figure 2: CT scan of patient #2, showing colitis extending from the cecum to the sigmoid colon. View Figure 2
We presented two very similar cases of unexplained colitis occurring within 2 months after laparoscopic BPD in two relatively young female patients. Neither patient has had previous attacks of colitis or any clinical clues indicating inflammatory bowel disease. In both cases, the clinical presentation and the laboratory tests raise the suspicion for an infectious etiology for the colitis. This hypothesis is further supported by the quick response to antibiotic treatment in both cases.
Derived from BPD's main mechanism of action, fat and bile acid malabsorption unanimously occur after the operation. Carbohydrate malabsorption can also occur. We hypothesize that the higher loads of fat, carbohydrates, and bile acids reaching the colon following BPD may render the colon susceptible to insults. This may explain the occurrence of colitis for the first time in two immunocompetent middle-aged females following BPD.
In 2008, Stijn Van Gool, et al. reported a case of Cytomegalovirus (CMV) colitis in an apparently immunocompetent host after bilio-pancreatic diversion for obesity [4]. A state of malnutrition in the reported patient was thought to be a predisposing factor. In addition, the authors suggested that some of the consequences of BPD, specifically exposure of colonic mucosa to excessive bile salts and bile acids, could have caused mucosal damage, predisposing for CMV infection. Apart from this case report, we found no publications in the English literature describing colitis as being a complication of BPD.
Several reports provided evidence for the influence of dietary fat on the composition of the colonic micro-flora. In a report by Marie A. Hildebrandt, et al. it was found that switching to a high fat diet in Murines caused a dramatic change in their colonic micro-flora, most prominently a bloom of Clostridia and Proteobacteria species [5]. It was shown that this shift in the gut microbiota was due to the high fat content reaching the colon, and not to the obese state itself. The diversity in gut microbiota is established since infancy, according to the predominant source of nutrition, as reported by De Filippo, et al. who found a great difference in the gut microbiota composition between European and rural African children [6].
An additional report showed that this alteration of microbiota can occur in a very short time, even within one day of switching from a low-fat diet to a high-fat/high-sugar "Western" diet [7]. The diet-induced shift also caused a change in metabolic pathways in the microbiome and altered microbiome gene expression.
Bile acids have potent antimicrobial properties illuminating many pathogenic and potentially harmful colonic bacteria. However, some intestinal pathogens, such as Bilophila wadsworthia, Helicobacter hepaticus and Listeria monocytogenes, are resistant to the antibiotic effects of bile, and are even selectively favored in a colon containing large amounts of bile acids which suppress the other commensal microorganisms. As a result, an imbalance in the microbiota can occur in conditions high bile acid loads reaching the colon, potentially contributing to inflammation and other pathologic states [8]. In addition, it is well established that dietary fat has a large influence on the turnover of Cholic Acid and on the composition of bile acids in humans [9]. A report by Riegler, et al. showed that luminal sodium deoxycholate causes epithelial damage of human colonic mucosa, and adversely affects the colonic mucosal cells' capability of migration and regeneration after injury [10].
Many reports provide strong evidence of the influence of gut microbiota composition on the immune function and pathologic processes in the colon. This composition influences the development and function of both innate and adaptive immune systems [11,12].
A report by Round, J.L. offered evidence that as a result of shifts in gut microbiota, some commensal, normally non-harmful, bacteria in the gut turn to be pathogenic; such bacteria are termed "pathobiont" [13].
A report by Elinav, et al. provided evidence that in cases of dysbiosis, i.e. alteration of the microbiota composition, an additional minor insult to the mucosal barriers, e.g. by toxins or diet, can result in an inflammation in the colon [14]. Such an inflammation would not have occurred following the same minor insult, if the microbiota preserved its normal composition. Pre-existing immunological impairment is not a prerequisite for dysbiosis-mediated inflammation. For example, treatment with antibiotics may shift the microbiota, causing outgrowth of pathogens such as Clostridium difficile, which can cause a clinically significant infection. According to the same concept, it is reasonable to assume that a shift in the microbiota, secondary to large gut fat content or alteration of the composition and concentration of bile acids reaching the colon, can render the colon susceptible to inflammation.
Some of the strongest evidence in support of this concept comes from the field of inflammatory bowel disease (IBD) research. A report by Frank DN, et al. provided evidence that the gut microbiota of patients with inflammatory bowel disease is often different from that of healthy people, particularly in the numbers of beneficial anaerobic microbes such as the Bacteroidetes and a subgroup of the Firmicutes. This proves that certain gut microbiota is required for the regulation of immune responses. As a result, alterations in the microbiota could disrupt this immunoregulation, causing an outgrowth of pathogenic microbes and the promotion of inflammation, particularly in genetically susceptible people [15]. Once the microbiota shift occurs, and pathogenic microbes outgrow, the by-products of these bacteria can cause mucosal barrier breakage, rendering the colon susceptible to deeper tissue damage.
Another factor that can influence the integrity of the colonic mucosa is the amount of carbohydrate reaching the colon. Taylor and co-authors reported 13 cases of noninfectious colitis associated with short gut syndrome in infants, attributable, at least in part, to the carbohydrate malabsorption that exists in these patients [16]. They suggested that excessive carbohydrates, which were unabsorbed more proximally and reached the colon, were metabolized by colonic bacteria to form elevated concentrations short-chain fatty acids, which are known to cause colonic mucosal damage [17].
Based on the aforementioned evidence and since gut fat content affects the composition of the microbiota, which in turn influences immune and inflammatory responses, then it is reasonable to conclude that gut fat content should have an effect on gut immunity and susceptibility to disease. This can be added to the insult to the colonic mucosa caused by the high bile acid and carbohydrate contents reaching the colon.
Whether this concept applies for the occurrence of colitis in the two reported patients is not clear, as specific examinations of the colonic microbiota of these patients were not performed. However, in the absence of clear malnutrition, vitamin deficiencies, or other derangements that can account for colitis, this possibility seems reasonable. Other potential causes for the colitis are IBD and ischemic colitis. The fact that, in both cases, a quick and complete response to antibiotic treatment was observed, favors an infectious, specifically bacterial, etiology for the colitis. The possibility of ischemic colitis does not seem appealing, as both patients are young and neither has predisposing factors such as vasculopathy or arrhythmias. The possibility of IBD also seems less favorable as neither patient had any suggestive symptoms prior to the reported attack, neither belongs to the high susceptibility age groups, and neither has relatives with IBD. Both patients subsequently underwent colonoscopy, showing no macroscopic or histologic evidence of IBD.
As we report 2 cases of a complication not previously reported, we suggest a high index of suspicion when BPD patients are re-admitted for abdominal pain.