Background: There is a widely acknowledged biological sex difference in anterior cruciate ligament (ACL) injuries, with females at a significantly higher risk compared to males. Due to the sex difference, the influence of the sex hormone estrogen has been investigated. Consequently, the aim of this study through reviewing current literature was to explore the association fluctuations in estrogen concentration during the menstrual cycle and altered ACL extracellular matrix (ECM) structure and subsequent function.
Methodology: Scopus and PubMed search engines were selected to perform a series of searches in April and May 2022. An exclusion criterion was implemented, and each article was critically analysed. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) structure was utilised to outline how the literature selection was made.
Results: Estrogen surges were concurrent with increases in knee laxity. A time delay of 2-3 days could be identified between the estrogen surges and increases in knee laxity, indicating the days that could hold the highest risk of injury due to ligaments being unable to tolerate normal loads. Moreover, specific to ACLs, increasing estrogen concentrations are coupled with reduced ultimate tensile stress and linear stiffness. The mechanisms by which estrogen impacts ACL function are still unclear; however, it has been suggested that estrogen causes alterations to ECM structure either by decreasing type I collagen production and ACL fibroblast proliferation, or by reducing collagen crosslinking via lysyl oxidase inhibition.
Conclusions: The findings of this review highlight that increases in estrogen concentration are associated with structural and cellular changes within ACLs, specifically decreased type I collagen content and crosslinking. Subsequent increases in knee laxity indicate estrogen should be considered a potential risk factor for ACL injuries in female athletes.
Estrogen, Sporting knee injuries, Anterior cruciate ligament, Knee laxity, Extracellular matrix
Knee injuries account for 41% of all sporting injuries, with Anterior Cruciate Ligament (ACL) injuries, accounting for one fifth of such knee injuries [1]. Moreover, ACL injuries impact over two million people worldwide annually with an incidence of 68.6 per 100,000 person-years, thus, demonstrating the large scale impact [2-4]. A crucial risk factor of ACL injury is biological sex, with females having a 3-6 times higher risk of experiencing an ACL injury compared to males [5,6]. This review explores the impact of estrogen on the observed sex difference.
ACL injuries occur due to one of three main mechanisms: Direct, indirect, and non-contact, with non-contact mechanisms forming the majority (60-70%) of ACL injuries [7,8]. Typically associated with sporting activities although not exclusively, ACL tears account for 64% of knee injuries in cutting and pivoting sports such as football [7]. The short-term impacts of ACL injuries include reconstructive surgery and rehabilitation, which are associated with prolonged periods of reduced activity and quality of life, and significant financial cost [9]. Based on a conservative estimate that 15,000 primary ACL reconstructive surgeries are performed by the UK each year, the minimum cost of ACL reconstructive surgeries on the UK equates to £63 million per year [10,11]. Furthermore, the percentage of athletes who have had ACL reconstructive surgery and returned to their reinjure level is just 65%, with only 55% returning to competitive sport, indicating the deleterious effect of ACL injuries [12].
Additionally, the long-term effects include an increased risk of developing osteoarthritis (OA), with approximately 50% of patients with ACL injuries developing OA within 5-15 years of the initial injury [13]. Resulting from ACL injury, damage to the articular cartilage and underlying subchondral bone within the knee joint can occur. Kinematic changes at the knee joint, increased shear and compressive forces, as well as increased inflammation, are all factors which can contribute to such damage [13]. Risk factors for ACL injury have been separated into intrinsic and extrinsic factors; extrinsic factors include environmental conditions, playing surfaces and footwear whereas intrinsic factors include influences such as genetics, anatomic variables and biological sex [14].
As the majority of ACL injuries are non-contact, arguably the intrinsic differences between males and females could explain the reasons for the discrepancy [15]. Intrinsic risk factors of ACL injury have been categorized as neuromuscular, biomechanical, anatomic and hormonal [16]. As a result, this has led to the hypothesis that there is a hormonal effect which impacts ACL injury [17].
Female sex hormones, progesterone and estradiol (estrogen), follow a rhythmic pattern within the menstrual cycle, which is comprised of two phases (follicular and luteal) [18]. At days 1-6 of the menstrual cycle, levels of estradiol and progesterone are at their lowest, with estradiol concentration peaking at days 12-14, just prior to ovulation [17]. In addition, in the luteal phase (days 20-24), there is a second, lower peak in estradiol levels [17]. At days 19-24 (the mid-luteal phase), progesterone levels are at their highest having risen gradually from the late follicular phase just prior to ovulation [17].
The effects of hormones on the musculoskeletal system have been investigated, and receptors for estrogen, progesterone and relaxin have been found in human ACL tissue, possessing varying roles in ligament metabolism [19-21]. The association between ACL laxity, a term denoting ligament flexibility and thus the ability of the ligament to withstand loads, and fluctuating hormone levels has been examined, identifying the phases of the menstrual cycle where a female could have a higher risk of injury [22,23]. Some have suggested that the pre-ovulatory stage of the follicular phase, when estrogen levels peak, is the period of highest risk of ACL injury due to increased ACL laxity [24].
The steroid hormone, estrogen, is found in three major physiological forms: Oestrone, estradiol and estriol, with estradiol the most abundant during reproductive years and most potent [18,25]. In addition to being essential for reproductive tissues, estrogen possesses roles in the skeletal, cardiovascular and central nervous system. Within bone remodelling, estrogen plays a key role in the balance between bone resorption by osteoclasts and bone formation by osteoblasts via the expression of certain cytokines and growth factors [26].
Cyclical raises in serum levels of estradiol have been identified as being responsible for increased knee laxity [27]. Moreover, fluctuations in estrogen concentration have been associated with changes to the Extracellular Matrix (ECM) composition of the ACL. ACLs are a band of dense connective tissue, comprised of collagen bundles (predominantly type I), elastin, glycosaminoglycans and proteoglycans. Raised estrogen has been linked with decreased lysyl oxidase (LOX) activity which catalyses collagen and elastin cross-linking, thus decreasing the stiffness of the ligament [28,29]. Therefore, understanding the mechanisms by which estrogen alters ACL structure and function is important when considering the increased ACL injury risk in females.
Thus, this review aims to elucidate the influence of estrogenon ACL ECM composition and subsequent functional changes.
Literature searches were performed during April and May of 2022. Scopus and PubMed were selected to conduct the literature searches (Figure 1). The combination of Scopus and PubMed ensured the most up-to-date and relevant research articles were identified, through the usage of advanced search criteria and exclusion functions.
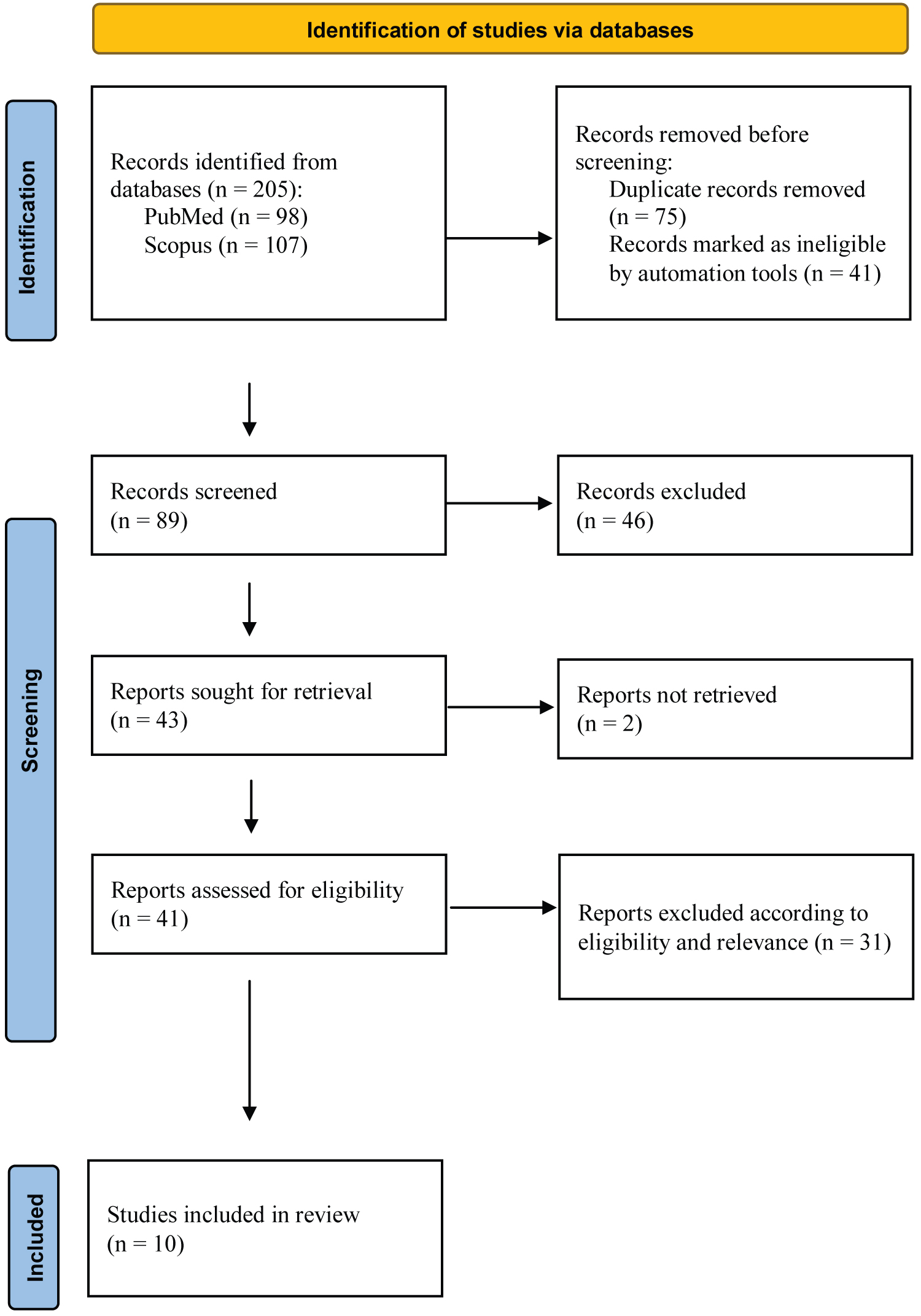 Figure 1: Adapted PRISMA diagram detailing the process by which the final publications were selected. Adapted figure [30].
View Figure 1
Figure 1: Adapted PRISMA diagram detailing the process by which the final publications were selected. Adapted figure [30].
View Figure 1
Keywords were identified to conduct relevant literature searches surrounding the topic of estrogen and sporting knee injuries (Table 1 and Figure 1). This produced a total of 205 hits. The advanced search tool and exclusion function were then utilised to include only primary research articles, removing publications such as reviews, book chapters and conferences, leaving a total of 164 hits (Figure 1). End Note enabled publications to be exported into a Microsoft Excel; the duplications were identified via the Excel search tool, “Find and Select” and then “Find” and removed leaving a final total of 89 hits (Figure 1).
Table 1: Literature searches performed. The numbers represent the order in which the searches were performed. View Table 1
Articles were excluded due to their focus on periodontal, uterosacral ligaments and spinal ligaments, as opposed to ACLs (n = 31). If the paper focussed on the impact of ACL reconstruction as opposed to pre-injury or the time of injury itself (n = 2) these were also excluded. Additionally, articles published prior to 2000 having been superseded and those conducted via a questionnaire as opposed to an experimental laboratory design were excluded (n = 13). If full access to a paper could not be reached due to the requirement of costly membership, then the publication was excluded (n = 2). Of the remaining 41 articles, 10 articles were shortlisted (Table 2), demonstrating greatest relevance to this study, indicating specific interest in estrogen, and meeting the requirements of the exclusion criteria. A challenge when conducting the literature search was the relatively small amount of literature currently available.
Table 2: Table containing a synopsis of the 10 shortlisted publications. View Table 2
Within the literature, it is widely recognised that fluctuations in female sex hormones, particularly estrogen, correspond with changes in knee laxity. This is important with regards to sporting injuries, given the implications laxity has on knee stability and the ability to withstand load. However, Belanger, et al. (2004) and Hertel, et al. (2006) found there was no significant correlation between changing hormone concentrations in the menstrual cycle and knee laxity and neuromuscular control, thus, there are differing research findings as to the effect estrogen has on knee laxity [39,40]. Additionally, challenges are created when comparing and critically analysing articles due to differing descriptions utilised to distinguish the phases of the menstrual cycle.
Both Shultz, et al. (2005) and Shagawa, et al. (2021) assessed the hormonal influence on knee joint function throughout the menstrual cycle in humans , and concluded that higher estradiol concentrations are concomitant with increased knee laxity [27,33]. Shagawa, et al. (2021) utilised a range of measurements: Anterior knee laxity, stiffness, genu recurvatum and general joint laxity, to identify the fore mentioned relationship [33]. Shultz, et al. (2005), in addition to identifying the link between hormone fluctuations and altered knee function also demonstrated the sex difference in knee function and hormone concentration fluctuations [27].
Shultz, et al. (2005) utilised an inclusion criterion which excluded those who had external hormonal intervention that could impact estrogen levels, such as through oral contraceptives or other hormone stimulating medication [27]. Moreover, those with prior knee joint injury or medical condition affecting the connective tissue of the joint such as Ehlers-Danlos disease (joint hypermobility syndrome) were also excluded, as anterior knee laxity may have been compromised by such conditions. This left 22 eumenorrheic (normally menstruating) females (23 ± 3.5 years-old), and with an adapted inclusion criterion and20 males (23.3 ± 2 years-old). 5-7 cm of venous blood was withdrawn at the same time of day to account for diurnal hormonal fluctuations, from females over the course of 20 days and from males over 4 test days. In females, beginning and end data collection was decided either as ovulation, via the detection of luteinizing hormone, or at menses onset, via self-report. Assays detecting estradiol, progesterone and testosterone were performed and in males, over the 4 days there was no significant difference in estradiol (p = 0.98), progesterone (p = 0.385) and testosterone (p = 0.143), thus an average was taken from the 4 measurements and utilised as a comparison to the 20 measurements from females. Estradiol levels were significantly greater (p < 0.0001) in females compared to males with said differences being cycle dependent. Due to males producing a lower and constant amount of estradiol, the influence on knee function provides an effective comparison when identifying the impacts of fluctuating estradiol concentrations in females. The phases that females had significantly higher estradiol concentrations were the days near ovulation representing the estradiol surge, the early luteal phase, and the late luteal phase.
Utilising the KT-2000 knee arthrometer, anterior knee laxity was measured and demonstrated comparable patterns to estradiol concentrations throughout the menstrual cycle, with females having significantly higher knee laxity than males (p = 0.023) and laxity values gradually rising following the estradiol surge. The differences in knee laxity that were significant between males and females throughout the cycle were similar to those significant differences in estradiol concentration: Days 3-5 near ovulation, early luteal days 1-4 and days 1, 2, 4 and 5 of the late luteal phase. Arguably, the data demonstrates a 2-3 day delay in response to estradiol concentrations, which may be important when considering times when individuals will be most prone to sporting knee injuries.
The ACL is critical in maintaining stability within the knee as it provides 87% of the total restraining force against anterior tibial translation [41]. Thus, alterations to ACL structure and function will have potentially deleterious implications to knee joint function. Wojtys, et al. (2002) identified that susceptibility to ACL injury in females was higher in the ovulatory phase of the menstrual cycle where, through urine samples, a peak of estrogen was detected [34]. The relationship between estrogen concentration and tensile load of the ACL was originally elucidated by Slauterbeck, et al. (1999), who utilised a rabbit model to demonstrate that increased estrogen concentration, reduces the tensile strength [42].
Komatsuda, et al. (2006), performed an ex vivo study also utilising a rabbit model, with the aim of identifying the relationship between ACL mechanical properties and estrogen concentrations to elucidate the sex difference in ACL injuries [35]. The rabbits were ovariectomized and then separated into groups according to the concentration of estradiol administered via intramuscular injection. Via mechanical testing, a statistically significant difference (p = 0.04) was found in the ultimate tensile strength between the medium (109 MPa) and high (69 MPa) estradiol groups which was the result of ultimate failure load divided by the cross-sectional area. Increasing serum estradiol corresponded with a reduction in ultimate tensile strength and linear stiffness, however, this association was not statistically significant (p = 0.07 and p = 0.06 respectively) and may indicate limits to the impact of estrogen on ACL mechanical properties. The clinical relevance of use of the rabbit model should be questioned, the rabbit ACL has been described as being anatomically different from human ACLs [43].
Liu, et al. (1996), localised estrogen receptors within the synoviocytes and fibroblasts in the ACL stroma and blood vessel walls of the ACL, thus indicating the potential cellular mechanism of estrogen within ACLs [44]. Collagen, specifically type I collagen constitutes around 80% of the dry weight of ligaments and is crucial to the mechanical strength of ligaments via molecular cross linking [45]. Research particularly focuses on the impact of estrogen on ACL fibroblast proliferation and subsequent ECM composition, with specific interest in collagen production. The inverse relationship between serum estrogen concentration, fibroblast proliferation and collagen production could explain the alterations in function and increased risk of injury of the ACL on certain days of the menstrual cycle.
In vitro studies : Yu, et al. (2001) utilised an in vitro model to identify the effects of variable estrogen and progesterone concentrations on ACL fibroblast proliferation and procollagen synthesis [37]. Precursors of collagen, known as procollagen, are markers of collagen biosynthesis. Per collagen molecule produced, one amino-terminal propeptide is cleaved from a procollagen molecule [46] . Hence, collagen biosynthesis can be quantified based on this assumption. Physiologic and supraphysiologic concentrations of estrogen and progesterone were replicated and ACL fibroblasts harvested from two patients suffering from different ACL injuries were exposed to varying hormone concentrations. By using 3H-thymidine to monitor actively dividing cells, fibroblast proliferation was monitored and a significant decrease in proliferation (p < 0.01) was seen with increasing concentrations of estradiol after day 1. This was contrasted with increasing concentrations of progesterone where fibroblast proliferation increased (p = 0.02). When the combination of estradiol and progesterone was administered on days 1, 3 and 5 there was a dose dependent decrease in proliferation (p < 0.01). However, such decrease in proliferation was prevented by increasing concentrations of progesterone and on day 7, there was no statistically significant correlation between fibroblast proliferation and estrogen or progesterone concentrations (p = 0.37). This suggests that progesterone possesses protective effects against estrogen induced decrease in fibroblast proliferation. This could be considered as a preventative measure to reduce incidence of knee injuries during phases of elevated estrogen levels.
Radio immunoassays were used to measure procollagen type I and III levels and such experiments revealed that type I procollagen synthesis followed similar trends to ACL fibroblast proliferation: Decreasing procollagen synthesis, with increasing estradiol concentrations. Furthermore, type I procollagen synthesis decreased significantly (p < 0.01) in a dose dependent manner on days 1, 3 and 5 with increasing estradiol concentrations, with such effects being attenuated by increasing progesterone concentrations. There was no significant effect on type III procollagen by either estradiol (p = 0.38) or progesterone (p = 0.41) and there is currently no evidence for its role when considering cyclical changes in knee function.
In vivo studies-rats: Yoshida, et al. (2009), performed an in vivo study utilising 12-week-old female Wistar rats to determine whether estrogen receptor (ER) location and expression of type I and type III collagen, and cartilage oligomeric matrix protein (COMP) in ACLs is related to changes in serum estrogen concentration and subsequent changes in ACL function [38]. The rats underwent ovariectomies and hormone replacement through subcutaneous implantation of a sustained-release pellet containing estradiol and/or progesterone. Knee joints were harvested 30 days after treatment and via immunofluorescence, ERα, ERβ, type I collagen, and COMP expression were quantified. Compared to the ovariectomy group, ERα expression was higher in the estradiol group as demonstrated by the higher volume of ERα-immunoreactive cells than in the ovariectomy group. Type I collagen immunoreactivity was significantly reduced (P < 0.05) in the proximal part of the ACL, in the estradiol group. Moreover, COMP expression was also significantly reduced in the estradiol group compared to the ovariectomy group (p < 0.05). COMP is known to be involved in collagen secretion and fibrillogenesis and interacts with multiple ECM proteins, thus reduced expression could be associated with decreased strength [47]. In the middle portion of the ACL, no significant differences were seen in the levels of type I collagen and COMP.
Interestingly, the morphology of the fibroblasts differed in the middle part (ovoid or spindle shaped) of the ACL compared to the proximal part (round) which may highlight the observed differences in type I collagen and COMP expression discussed above. Subsequently, Yoshida, et al. (2009) perhaps demonstrates that estrogen impacts ACL ECM composition in a localised fashion which should be acknowledged when considering susceptibility of certain parts of ACLs to injury specifically, the proximal part.
As in Yu, et al. (2001), Yoshida, et al. (2009) demonstrated the protective effects of progesterone, as the combination of estradiol and progesterone resulted in a lack of significant difference in type I and COMP expression compared to the ovariectomy group [37,38].
In vivo studies-humans : In a preliminary study, Shultz, et al. (2012), aimed to identify the effects of alterations in sex hormone concentrations across the menstrual cycle on both knee laxity and type I collagen metabolism [32]. They also hypothesised that cyclic changes in collagen metabolism could be associated with cyclic changes in anterior knee laxity. Healthy, eumenorrheic females were compared to females using oral contraceptives. Assays measuring sex hormones including estradiol, and serum markers of collagen synthesis and degradation (C-terminal propeptide of collagen type-I (CICP) and insulin growth factor-1 (IGF-1)), and collagen degradation marker (Carboxyterminal telopeptide of type I collagen (ICTP)) were measured. Prior to ovulation, the estrogen surge on days corresponded with decreased ICTP levels and were significantly lower (p < 0.05) than in the oral contraceptive users. CICP concentrations were significantly decreased (p < 0.001) in the early luteal and late luteal phases in both groups compared to in the days of menses and estrogen surge. In eumenorrheic females, IGF-1 levels remained stable (p = 0.558), whilst there was a decrease in the luteal phase (p < 0.001) in oral contraceptive users who therefore had lower IGF-1 levels than eumenorrheic females. Anterior knee laxity remained stable in oral contraceptive females (p = 0.429) but increased in the early and late luteal phase in eumenorrheic females (p = 0.029). Importantly, bivariate correlations, taking into consideration patterns of variability, revealed that decreases in serum CICP and increases in serum IGF-1 could predict anterior knee laxity increases in both groups (p = 0.014).
The results of Shultz, et al. (2012) only partially support their hypotheses that concentrations of serum collagen markers and mediators would be lower when estradiol levels are elevated [32]. Across the menstrual cycle, IGF-1 concentrations did not change in eumenorrheic females, which could suggest the interplay between estrogen and testosterone, given estradiol is known to decrease IGF-1 responsiveness and testosterone elevates IGF-1 concentration [48]. The pattern of IGF-1 seemed to follow that of testosterone concentrations in eumenorrheic females. Additionally, despite suppressed IGF-1 concentrations in oral contraceptive users, cyclic changes in anterior knee laxity were not observed. This is surprising given that low IGF-1 has been related to smaller collagen fibril diameter and diminished collagen synthesis which could be indicative of a poorer collagen and subsequent ACL structure [49].
The results of Shultz, et al. (2012) also demonstrate that decreased CICP and increased IGF-1 are predictors of increased anterior knee laxity in both groups, which perhaps suggests that CICP and IGF-1 interact, leading to structural changes in ACLs (Figure 2) [32]. This is deduced from the raised anterior knee laxity in the estradiol peak prior to ovulation in eumenorrheic females, following the suppression of CICP and increase (although not significant) in IGF-1. The lack of anterior knee laxity cyclical changes in the oral contraceptive females may be due to the constant lower levels of estradiol.
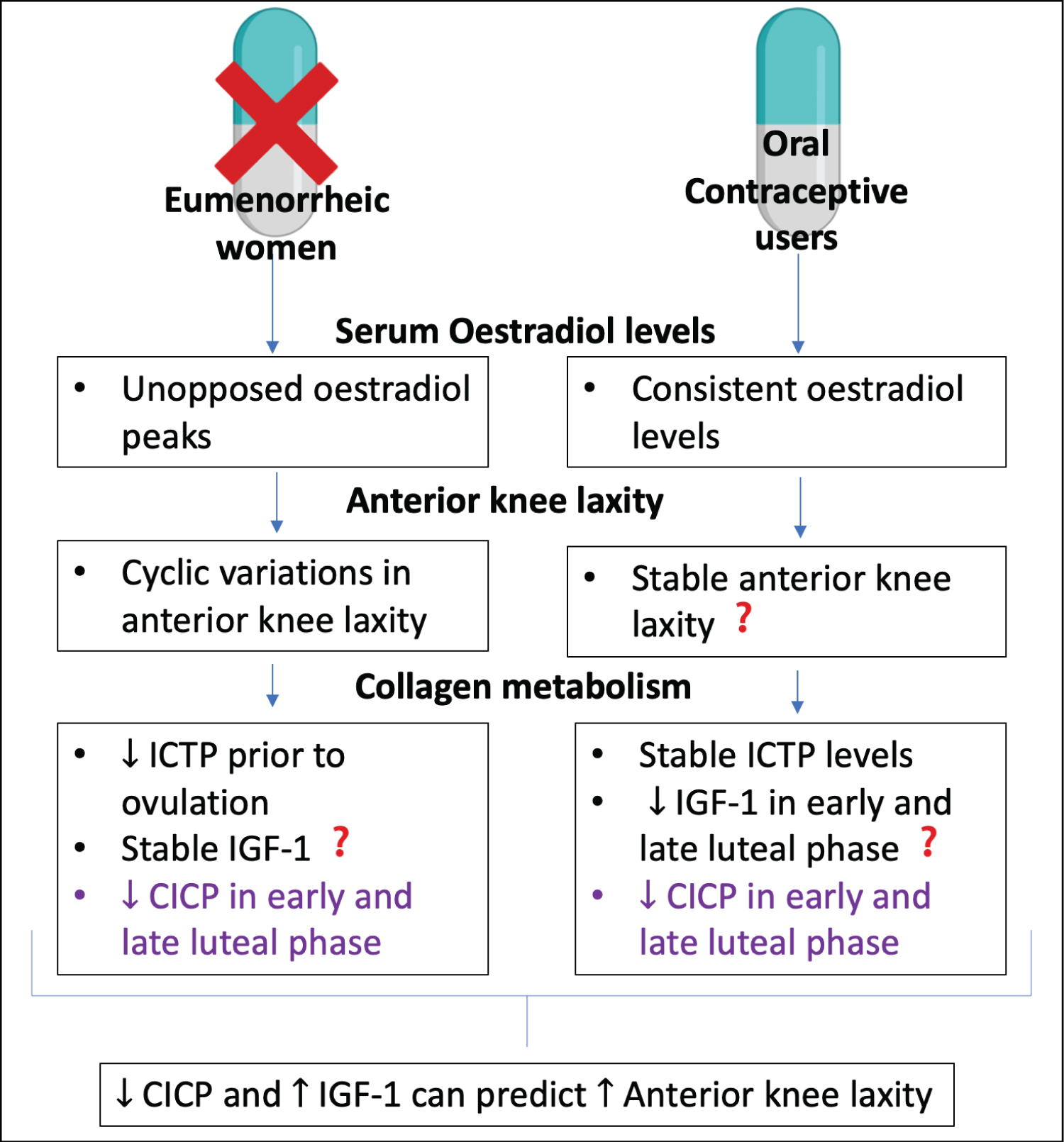 Figure 2: A summary of key results from Shultz, et al. (2012) [32]. Comparison between the results of eumenorrheic (normally menstruating) and oral contraceptive users are presented, with regards to serum estradiol levels, anterior knee laxity and collagen metabolism. From the results, bivariate correlations revealed that decreased CICP and increased IGF-1 can predict increased anterior knee laxity. Results that were not expected or went against the authors hypothesis are followed by?. In purple is a result seen in both groups. Original diagram made using Biorender.com.
View Figure 2
Figure 2: A summary of key results from Shultz, et al. (2012) [32]. Comparison between the results of eumenorrheic (normally menstruating) and oral contraceptive users are presented, with regards to serum estradiol levels, anterior knee laxity and collagen metabolism. From the results, bivariate correlations revealed that decreased CICP and increased IGF-1 can predict increased anterior knee laxity. Results that were not expected or went against the authors hypothesis are followed by?. In purple is a result seen in both groups. Original diagram made using Biorender.com.
View Figure 2
Lastly, Shultz, et al. (2012) demonstrated that fluctuations in sex hormones, particularly estrogen, across the menstrual cycle influence type I collagen metabolism and anterior knee laxity [32]. The authors begin to suggest that such change in collagen metabolism could affect ACL structure and function; although due to the complex interplay of sex hormones and mediators of collagen synthesis and degradation, this requires further research. With regards to this study, the understanding that cyclical decreases in CICP and increases in IGF-1 could predict anterior knee laxity, demonstrates that perhaps, estrogen influences collagen metabolism.
Whilst prior research has focused on the impact of estrogen on the composition of the ACL ECM, the action of the enzyme LOX has been suggested to be important in the structural integrity and thus, function of the ACL. LOX is known to catalyse the cross linking of collagen and elastin, and is therefore crucial in ECM formation and the structure of the ACL [28]. Consequently, Lee, et al. (2015) hypothesised that estrogen alters ACL function by reducing LOX activity rather than altering ECM composition [29]. Research into LOX and ACLs mainly focusses on the healing capabilities of the ligament, given that LOX is also associated with wound healing. Xie, et al. (2013) demonstrated that Medial Collateral Ligament (MCL) fibroblasts had higher expressions of LOX than ACL fibroblasts which could explain the poor healing qualities of the ACL [50].
Lee, et al. (2015) hypothesised that estrogen decreases the mechanical properties of ligaments via inhibition of LOX, utilising engineered ligaments constructed from ACL cells [29]. Mechanical testing by a custom-built tensile tester revealed the maximal tensile load (MTL) and derived ultimate tensile strength (UTS) of the engineered ligaments treated with estrogen concentrations ranging from low (5 pg/ml), medium (50 pg/ml), and high (500 pg/ml). The UTS was significantly decreased (p = 0.02) following 48 hrs of exposure of high estrogen concentration which mimicked the peak of estrogen prior to ovulation. However, collagen content did not decrease; thus, the authors proposed the importance of differing cross-linking activity. An assay revealing LOX activity, revealed that ligaments treated with high estrogen concentration for 24 hrs resulted in a 62% decrease in LOX activity (p = 0.0084). Likewise, when treated for 48 hrs, there was a 77% decrease in activity (p = 0.0021). Total cellular RNA measurements indicated LOX gene expression decreased by 25% (p = 0.03) after 24 hrs of exposure to high estrogen levels. To then assess whether LOX impacts the mechanical properties of the ligaments, the ligaments were treated with the LOX inhibitor β-Aminopropionitrile (BAPN). This led to a significant decrease in MTL and UTS (p < 0.05). Consequently, Lee, et al. (2015) proposes an alternative mechanism of action by which estrogen alters ACL activity suggesting that it might not be the collagen content, rather the arrangement and crosslinking of the collagen that impacts mechanical function.
This review explored the association between estrogen and changes in ACL structure and function. This association is important in understanding the mechanisms behind the sex difference in ACL injuries. Although, the hormonal effect is commonly acknowledged in literature, the mechanisms by which fluctuations in estrogen and other sex hormones impact knee joint structure and function are not fully understood. There are a limited number of preliminary studies aiming to elucidate this relationship and further research is needed to determine how estrogen can be considered a risk factor in sporting knee injuries and whether there are any preventative measures that can mitigate such effects.
Shultz, et al. (2005) indicated that increases of estrogen concentrations throughout the menstrual cycle correspond with increases in knee laxity [27]. This alteration in knee laxity may demonstrate days during the menstrual cycle when females are less able to withstand high loading through the knee, and thus, are more susceptible to sporting knee injuries. Komatsuda, et al. (2006) also demonstrated in rabbits that increased estrogen has an impact on ACL function, indicated by the significant decrease in UTS [35]. Arguably, it is the unopposed estrogen surges that present the risk factor to knee injuries, as Yu, et al. (2001) and Yoshida, et al. (2009) demonstrated both in vitro and in vivo respectively the protective effect of progesterone against estrogen mediated changes [37,38]. Shultz, et al. (2012) compared knee laxity and collagen metabolism in eumenorrheic females and oral contraceptive users [32]. This comparison presented the opportunity to observe the effect of the estrogen surges within the menstrual cycle, as those taking oral contraceptives had consistent estrogen levels without surges that were matched by consistent progesterone levels. There was a lack of cyclic variation in knee laxity in the females taking oral contraceptives, thus suggesting the negative impacts of estrogen surges in eumenorrheic females. Additionally, research indicates that the peptide hormone relaxin, acts synergistically with estrogen, exerting collagenolytic effects, thus altering ACL function and should be taken into consideration [51].
Yu, et al. (2001), Yoshida, et al. (2009) and Shultz, et al. (2012) explored how estrogen alters collagen metabolism and as a result, it could be argued that estrogen exposure alters ACL function by decreasing ACL fibroblast proliferation and type I collagen production [32,37,38]. However, it could be argued that alterations in rates of collagen synthesis over the short time frame of the estrogen surges within the menstrual cycle could be of a too small a magnitude to have an overall effect on knee function. The turnover of collagen is known to be low in ligaments: Between 100 to 500 days [52]. Moreover, Smeets, et al. (2019) identified that the tissue protein synthesis rate within ACLs is 0.07 ± 0.02%/hr, which would suggest that alterations to ECM composition over the short time frame being considered may be too small to significantly raise the risk of knee injury [53]. Additionally, Seneviratne, et al. (2004) aimed to identify the effect of estrogen on ovine ACL fibroblasts and identified that there was no significant difference in ACL fibroblast proliferation or collagen synthesis [52].
Consequently, Lee, et al. (2015) proposes a mechanism by which estrogen alters ACL structural integrity without necessarily changing the protein composition that could be more plausible [29]. The enzyme LOX is crucial in catalysing collagen and elastin cross links by oxidising lysine residues, and therefore is important when considering the ECM mechanical function [54]. Lee, et al. (2015) demonstrated that estrogen exposure in engineered ligaments led to significantly decreased LOX activity and such decreased activity was associated with decreased mechanical function of the ACL [29]. This decrease in mechanical function was also demonstrated when a LOX inhibitor was added, thus indicating the importance of LOX activity when considering ACL function. In addition, Wang, et al. (2020) suggested that via the nuclear factor-kappa B pathway, LOX promotes tissue regeneration in ACL fibroblasts via the suppression of TNF-α, which suggests that LOX also modulates the inflammatory response in ACLs which is important when considering the high stresses ACLs are under in sporting environments [55]. In current literature, research into periodontal ligaments and uterosacral ligaments of those suffering from pelvic organ prolapse, concurs with the hypothesis that expression and activity of LOX relates with ligament function. Therefore, when considering risk of sporting knee injury, LOX activity and how estrogen may alter such activity should be researched into further.
There are gaps in current understanding surrounding the role of estrogen in ACL structure and function and subsequent injuries. Current research implicates estrogen with the upregulation of osteogenic differentiation in periodontal ligaments [56]. Such alterations in cellular phenotype result in bone formation and thus structural changes to the ligament. Consequently, future research could consider if similar effects on osteogenic differentiation occur in ACLs due to changing estrogen concentrations. Additionally, this paper has highlighted the potential importance of LOX activity; however, the mechanisms by which estrogen alters such activity in ACLs remain unclear. Current literature indicates that specific micro-RNAs alter LOX activity and ER expression in females with pelvic organ prolapsed [57,58]. Thus, future research into micro-RNA content and activity in ACLs should be considered, as this could demonstrate an alternative mechanism by which individuals could be more susceptible to injury. Lastly, future research should consider potential preventative measures, with more studies needed to look at the usage of oral contraceptives by female athletes.
During the menstrual cycle, unopposed surges in estrogen occur, such increases in concentration have been linked with altered ACL ECM structure which in turn, decreases the mechanical function of the ACL. Overall, this is associated with decreased knee function due to increased knee laxityand thus potential increased risk of sporting knee injury in females.In grey are proposed mechanisms by which estrogen effects ECM structure, however the extent to which each mechanism plays in altering knee function is not conclusive . In yellow are areas that should be considered for future experiments both with regards to mechanisms of action by which estrogen impacts ACL structure, and preventative measures such as use of oral contraceptives. Original figure made using Biorender.com (Figure 3).
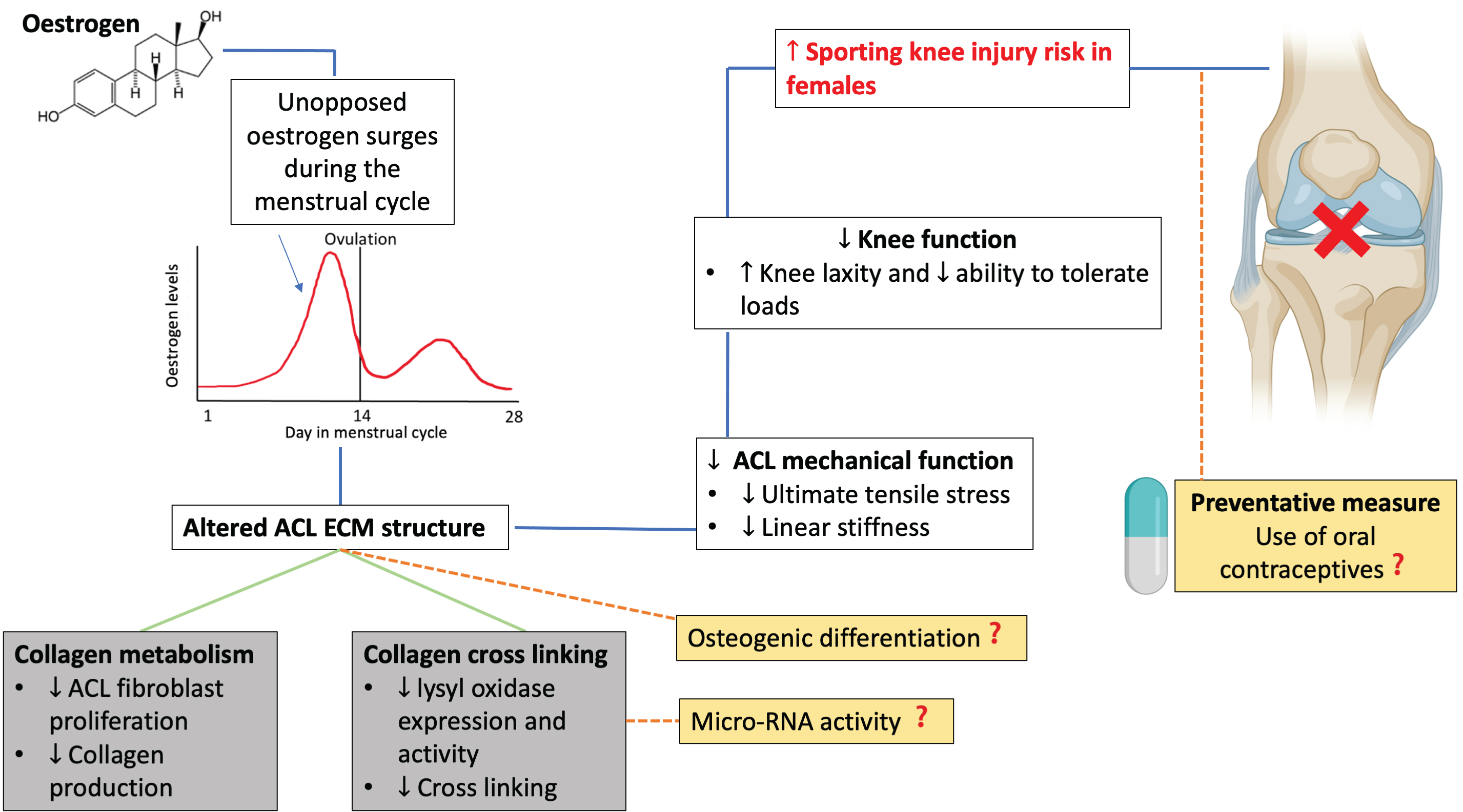 Figure 3: A schematic representation of the proposed mechanisms by which estrogen impacts knee function and thus, in females, is a risk factor in sporting knee injuries.
View Figure 3
Figure 3: A schematic representation of the proposed mechanisms by which estrogen impacts knee function and thus, in females, is a risk factor in sporting knee injuries.
View Figure 3
Overall, by reviewing current literature that addresses the association between estrogen and alterations in ACL structure and function, the mechanism of action appears to be involved with type I collagen metabolism and/or crosslinking. Increases in estrogen concentration throughout the menstrual cycle should therefore be considered an important risk factor for female athletes with regards to ACL injuries.
The authors report there are no competing interests to declare.