Background: A new test for assessing supraspinatus muscle (SM) injury, the Diagonal Horizontal Adduction test (DHA), has been developed which demonstrates more isolation of the SM and the similar widening of cross-sectional area (CSA) visualized through musculoskeletal ultrasound (MSK).
Purpose: The purpose of this study was to determine whether the resistance changes in CSA of the SM using DHA testing are consistent with the forces generated by this muscle under voluntary contraction using Isometric Dynamometry (ID).
Study Design: A prospective cohort study involving 44 subjects (mean age of 24.2 ± 1.9) with no previous history of shoulder surgery or pathology. All subjects performed graded resistance and no controls were necessary.
Methods: Subjects were observed and measured while providing isometric contraction in the DHA position using Biodex ID during an isometric contraction at resistance levels of 0%, 50%, and 100% of maximum contraction. Cross-Section Area (CSA) of the SM was captured and measured using a Terason t3200 MSK ultrasound unit.
Results: One-way Analysis of Variance with repeated measures examining differences of CSA at each level of resistance were significant at p < 0.05. Post-hoc testing revealed a significant difference in CSA between 0% and 100%, 0% and 50%, and 50% and 100% maximal voluntary contraction of the muscle.
Conclusions: The CSA of the SM using MSK is consistent with the amount of force generated by the muscle during contraction. Greater force led to significantly greater CSA.
Clinical Relevance: The change in CSA during increased resistance suggests previous comparisons of SM isolation using the DHA technique are useful in determining strength of contraction and amount of activation during manual testing and may be able to be used in place of fine-wire EMG.
What this Study Adds to Existing Knowledge: The DHA test for the supraspinatus shows comparable results with CSA and MSK Ultrasound as compared to other SM testing methods such as local and fine wire EMG.
Musculoskeletal ultrasound, Cross-sectional area, Supraspinatus, Diagnostic testing
The glenohumeral joint is the most mobile joint in the human body and is responsible for the effective positioning of the upper extremity so that the hand can complete both simple and complex tasks [1,2]. This joint is also a common sight of lesion and functional loss, with shoulder problems affecting up to 67% of the population at some point in their lifetime [3,4].
The rotator cuff muscles have primary responsibility for shoulder stability [5]. The health of the rotator cuff is essential to normal functional activities as this group of muscles fixes the head of the humerus into the glenoid fossa [6]. The supraspinatus muscle (SM) is important for the stabilization of the glenohumeral joint and aids in abduction and external rotation of the humerus [6]. Dysfunction in the SM muscle leads to decreased stability of the GHJ as a whole [7]. Finding consistent means to assess the strength and function of the SM is important in developing new, and possibly more accurate, special tests.
The activity of the SM is traditionally examined in two ways. First, Cudlip, et al. [8] suggest the utilization of EMG, either via surface or fine wire, for testing to assess function and activity of the SM [8]. However, surface electrodes were determined to have some contamination in signal from surrounding structures, and fine wire electrodes are found to pick up electrical signal from only portions of the muscle [9]. Therefore, these commonly utilized methods of testing muscle activity are self-limiting.
Jobe and Moynes [10] suggested the Empty Can test, and Kelly, et al. [11] and Escamilla, et al. [12] suggested testing using the Full Can test, to reduce impingement and pain while maintaining the same level of activity of the SM. These two tests measured the strength and activity of the SM and have been shown to be reliable and valid tests of function [13]. Both the Empty Can test, and the Full Can test, both tested in less than 90o of abduction, have been verified through EMG as positions that effectively activate the SM muscle. However, other muscles (such as the deltoid and infraspinatus) were found to contribute to activity in these test positions [14]. Yasojima, et al. [15] suggested an angle near 60o of abduction was best for activation of the rotator cuff while also limiting contribution of the deltoid and infraspinatus muscles using these test positions.
A more recent test and position, the Diagonal Horizontal Abduction test (DHA), has been developed for SM testing [16]. The DHA test was shown to isolate the SM from other collaborative musculature better than either the Empty Can or the Full Can tests [16]. This test is performed with the shoulder in a diagonal horizontal adducted position and is presently referred to by the action needed to return from this position (diagonal horizontal abduction) (Figure 1). In this same study by Forbush, et al. [16], the SM was found to demonstrate significant change in cross-sectional area (CSA) while also demonstrating less activity of the deltoid musculature than either the Empty Can or Full Can testing. To date, no other articles published utilize or analyze this test position for SM activity. In addition, testing using EMG has not been utilized for the DHA test due to previously examined limitations of EMG tests for SM.
A second non-invasive technique used to analyze the activity of the SM is through the use of musculoskeletal ultrasound (MSK) and measurement of the cross-sectional area (CSA) of the muscle [17-20]. The MSK measurement is found to be as accurate as magnetic resonance imaging (MRI) in determining the CSA of the SM [17,19,20]. Using the gold standard assessment, MRI, two research groups demonstrated a favorable relationship between the CSA of a muscle to the strength and function of that same muscle [18,20]. Therefore, cross-sectional changes relative to resistance to a position can be used to assess activity and strength of a muscle group and has a high measure of inter-observer reliability [20].
A commonly used method to objectively assess muscle strength is through the use of isokinetic dynamometers (ID). These isokinetic devices test the muscle by providing constant velocity (even at 0o per second, or isometric) with accommodating resistance within any range of motion of the muscle [20,21]. Biodex ID has been used to obtain valid and reliable measures of torque and resistance [21]. Because this method of strength evaluation provides a more quantitative analysis than manual muscle testing, ID has become popular in both clinical and research settings [22]. However, ID does not allow an isolated analysis of a single muscle's contribution, as many muscles contribute to motions allowed within ID testing [22].
The alternative means of evaluating contraction of a muscle, such as the SM, through measurement of the changes of the muscles cross-sectional area (CSA) with diagnostic musculoskeletal ultrasound (MSK) has been analyzed [17,19]. Two groups of researchers, Grazioli and colleagues [23] and Juul-Kristensen, et al. [24] used CSA of muscle through MRI analysis and determined that CSA measurement changes could be used in determination of the strength and activity of the muscles of the rotator cuff. Further, Akagi, et al. [25] concluded that muscle CSA can be assessed as easily with diagnostic MSK as with MRI. Studies have shown that imaging with MSK, often used both clinically and in research to examine structures of the musculoskeletal system, is a reliable and valid means of evaluating SM CSA when compared with measurements obtained from MRI and computer-aided tomography (CT) [17,20,26]. Furthermore, MSK is more accessible and less expensive than MRI which is considered the gold standard for soft tissue evaluation, and less invasive than CT which exposes the patient to ionizing radiation [27].
The purpose of this study is to compare the CSA of the SM (evaluated through the use of MSK) to the torque as measured using ID. The CSA was measured during ID isometric resistance while performing of the new DHA special test. Comparing torque measurements collected through the assessments gathered through ID with changes in CSA readings collected by MSK, the researchers attempted to establish that CSA changes during the DHA testing procedure for the SM muscle accurately assesses the strength or activity of the SM.
The University Institutional Review Board approved the study. Forty-four subjects (x = 24.2 ± 1.9) were tested involving 29 females and 15 males. Handedness was determined by asking the subjects preference in writing and throwing behaviors.
The Biodex ID (Biodex System 4 Pro, Shirley, NY) was utilized for all resistance trials and activities. The set-up of the machine was able to approximate the position of DHA testing and the screen was able to be used for biofeedback to the participant to allow accurate resistance levels during testing.
The ultrasound unit used to collect MSK data was the Terason t3200MSK ultrasound machine (Terason Ultraound, Division of Tretech Corporation, Burlington, MA) with a 15L4 linear array transducer head. The MSK machine was set up on a table away from the participants so that the patient was unable to see the screen of the unit.
Data was collected at the SM while using the DHA testing technique in a Biodex ID and performing isometric resistance of 0%, 50%, and 100% of maximal contraction via MSK. The subjects were set into the ID with the dominant arm placed into horizontal adduction while at 60o of elevation and in full external rotation of the shoulder, so the upper arm was approximated to the nipple line of the chest on the dominant side.
The Biodex ID was set at a cross-body diagonal angle to accommodate for the Diagonal Horizontal Adduction (DHA) position so that each participant was at the same angle during all testing (Figure 1). To set this position, the Biodex was set at 35o tilt of the actuator, 30° rotation, and 30° of chair rotation. Patients were tested in a seated position and secured with two band across the chest, one across the pelvis, and one across the contralateral leg. The elbow was fully extended, and the shoulder was placed in horizontal adduction, 35o of flexion, and fully externally rotated at the shoulder with the thumb pointing anteriorly. The arm was placed across the nipple line of the near chest so that the triceps lay across the corner of the chest. The hand grip attachment was used in all three exercise trials. Gravity correction was applied. Participants were instructed to maintain an extended elbow during all tests. All test positions were conducted at the chosen range to optimize contraction without onset of potential impingement against the chest in the DHA position. Participants completed the shoulder movement against the isometric resistance of the Biodex ID and the computer-generated torque was measured and collected.
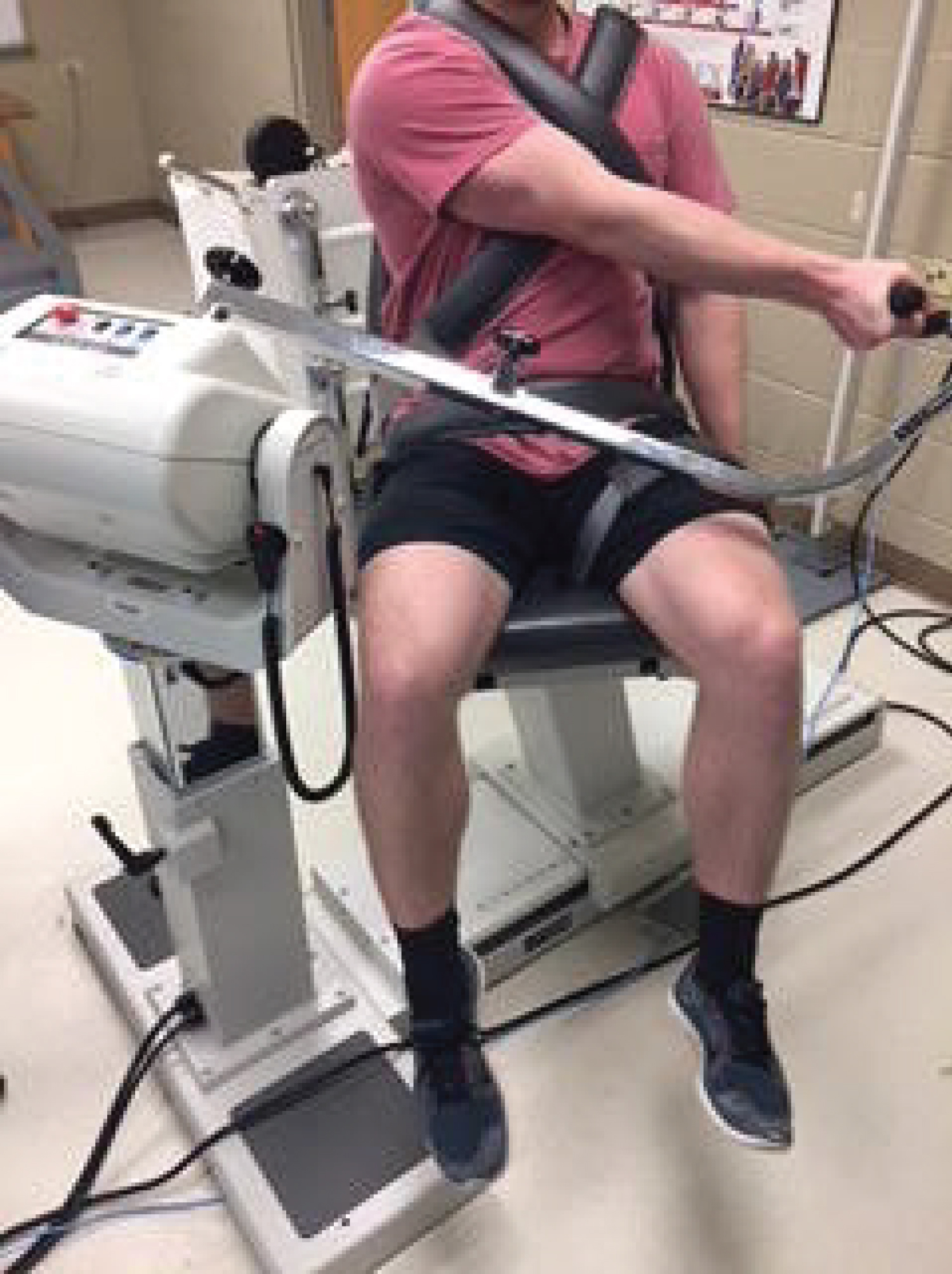 Figure 1: Test position of subjects on isokinetic dynamometer.
View Figure 1
Figure 1: Test position of subjects on isokinetic dynamometer.
View Figure 1
In the present study, the use of MSK to measure contraction using the DHA test of the SM was performed by one examiner trained in the use of diagnostic ultrasound for musculoskeletal conditions and with 10 years of experience. All CSA reading and measurements were made by the same investigator. The transducer head of the MSK unit was held perpendicular to the supraspinatus muscle at the location just inferior to the scapular notch in the sagittal plane (Figure 2). Optimal location at the thickest part of the muscle medial to the acromion was found. Through visual observation, the probe angle was adjusted based on the size and shape of the individual's muscle for the best image. Images were then captured (Figure 3). Images were analyzed using a consistent measurement of CSA of the supraspinatus at the scapular notch and recorded for each resistance level.
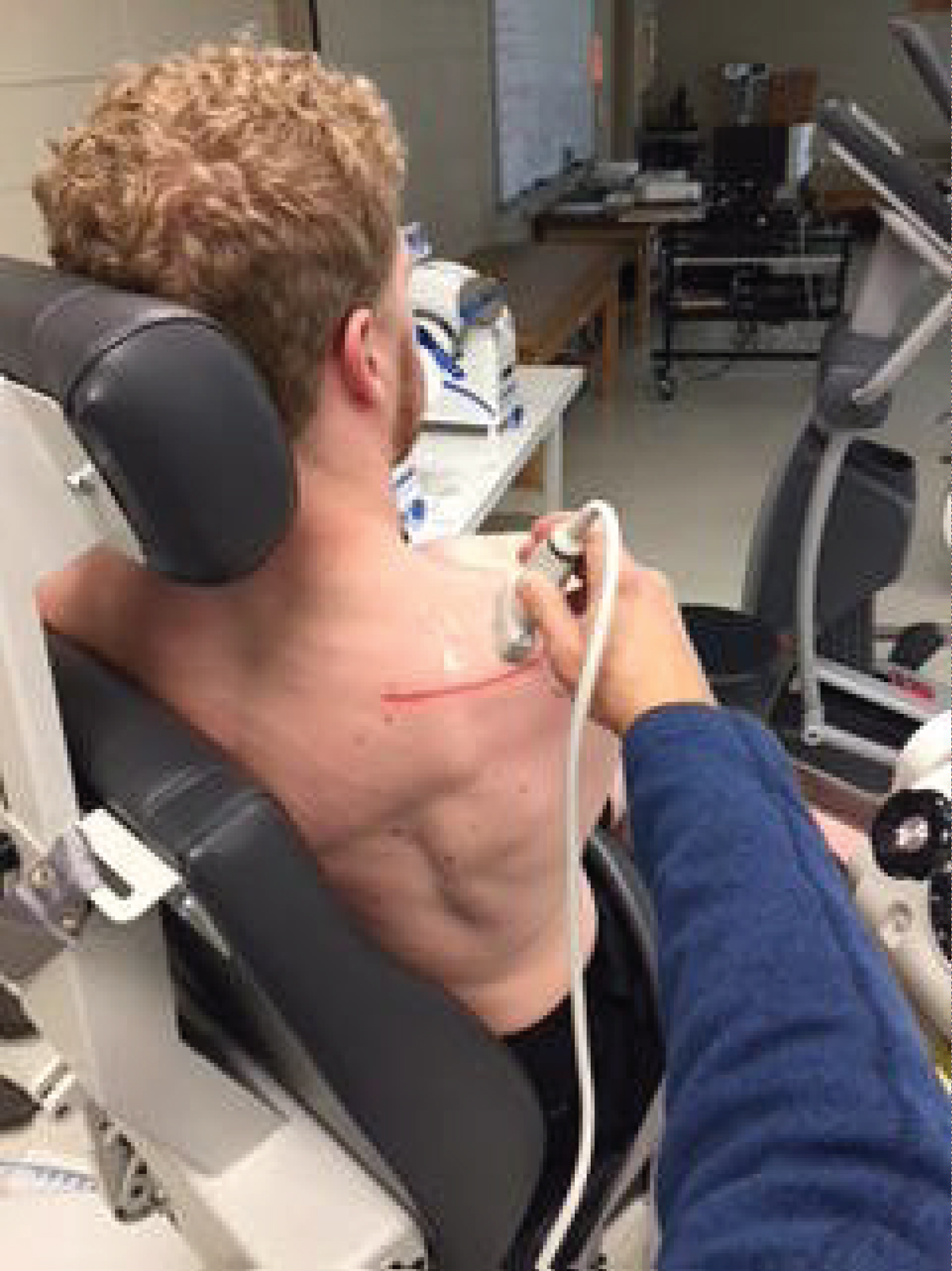 Figure 2: Position of ultrasound transducer of supraspinatus fossa.
View Figure 2
Figure 2: Position of ultrasound transducer of supraspinatus fossa.
View Figure 2
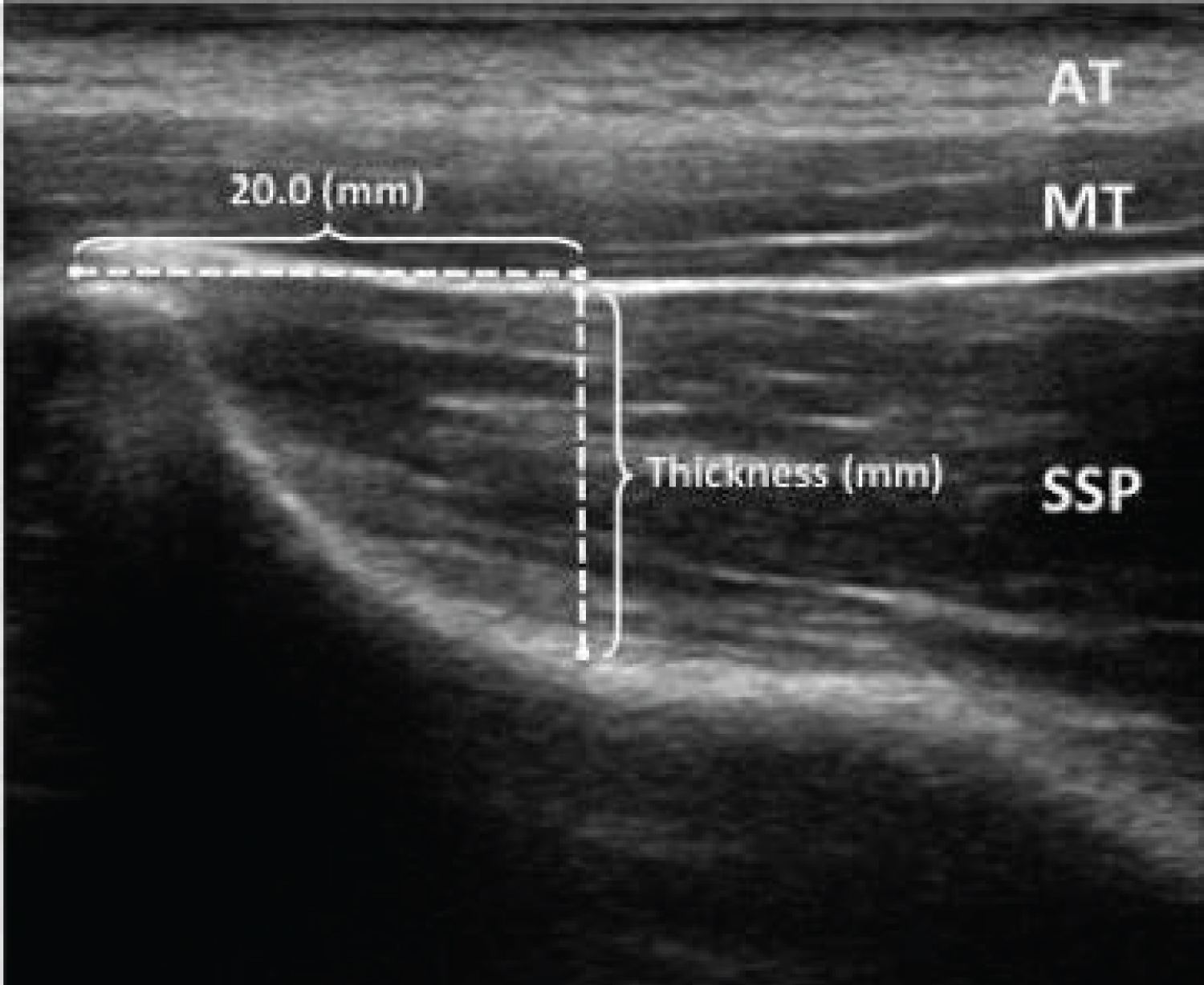 Figure 3: Ultrasound image and measurement of CSA.
View Figure 3
Figure 3: Ultrasound image and measurement of CSA.
View Figure 3
A resting MSK image was created before each contraction (0% maximal contraction). Each subject was then asked to produce three-five second contractions at 100% maximal volitional isometric effort. The subjects were then given a one-minute rest time between this set of contractions and the next set of contractions. The Biodex monitor was then digitally marked for the maximal contraction and for a 50% volitional contraction. The subject was then asked to produce three-five second contractions at 50% volitional contraction. The MSK images were captured for each contraction and were later marked and measured for CSA.
The mean and standard deviation of the CSA measurements obtained with each percent of isometric resistance (0%, 50%, and 100%) were calculated. The difference between means of the CSA of the SM and the contraction torque produced with the Biodex ID was analyzed through a repeated measure one-way ANOVA using SPSS version 22.0 statistical software (SPSS Inc., Chicago, IL) and post-hoc analysis was completed using pairwise comparisons utilizing least significant difference. Results of these analyses are presented in Table 1.
Table 1: Means of cross-sectional area with differing loads. View Table 1
The means of CSA of the SM during contraction through isometric resistance produced by the Biodex ID at 0%, 50%, and 100% of maximal voluntary effort by each of the subjects are presented in Table 1. Results of the one-way ANOVA indicated significance differences between the three groups (p < 0.5, F = 40.68) (Table 2). Post hoc analysis indicated significant difference in the CSA between all three groups (0%, 50%, and 100% maximal voluntary contraction of the muscle p < 0.05) (Table 3). The F-value was 40.68 and the differences were significant at p < 0.05. Results for the ANOVA and Wilks' Lambda are found in Table 1 and Table 2. Results of the post-hoc analyses are found in Table 3. As the resistance level increased, the CSA of the SM also significantly increased but the change was not linear as demonstrated in Figure 4.
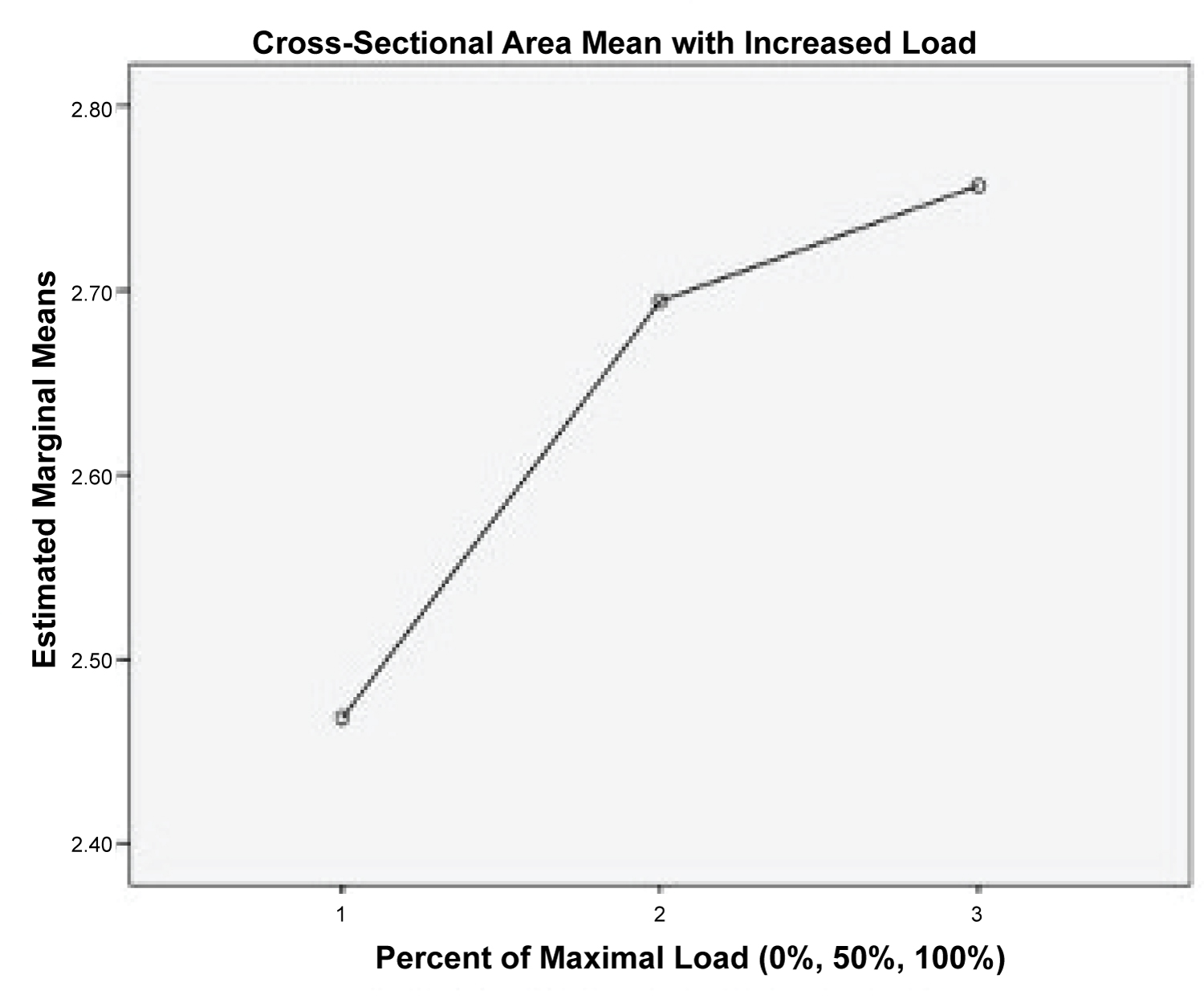 Figure 4: Graph of mean CSA with varying resistance.
View Figure 4
Figure 4: Graph of mean CSA with varying resistance.
View Figure 4
Table 2: Multivariate tests. View Table 2
Table 3: Within groups CSA effects of torque at 0%, 50%, 100%. View Table 3
In the current study, CSA measurements were taken to define activity while testing different resistance of the SM while performing the DHA test. Ultrasonography is used as a means of defining activity in a muscle is both sensitive and specific when used for identifying changes in morphology of the rotator cuff [28-31]. The purpose of this study was to determine whether the resistance changes in CSA of the SM using DHA testing are consistent with the forces generated by this muscle under voluntary contraction using Isometric Dynamometry (ID) [16].
Using the DHA test and MSK measurement of CSA, the SM was easily visualized. Differences in mean CSA were delineated between different levels of contraction and all levels of contraction were found to be significantly different when compared to no contraction. The increase in CSA with increased load from 50% to 100% was not purely linear and a lesser increase in CSA between 50% to 100% occurred when compared to changes from 0% to 50% (Figure 4). Suggestion could be made that changes in CSA of the SM using the DHA test, found in a previous study by Forbush, et al. [16], would indicate increased muscle activity with increased resistance. Also, differences in CSA would relate to level of resistance of the SM. This is consistent with findings for other testing of the supraspinatus including the FC and EC tests by other researchers [17,19,32].
The current study supports the work of previous studies stating the use of MSK to measure CSA volume changes within the supraspinatus muscle are reliable and valid compared to findings on MRI. Katayose and colleagues [17] established a reference standard for the CSA of the SM using 72 subjects across a wide variety of ages. The authors stated the CSA diminished with age and was larger in the dominant arm. In the present study, the dominant arm was tested, and all subjects were in the younger age categories making changes in CSA easier to visualize. In a study of a subset of 25 active individuals without pathology, Schneebeli and associates [19] compared MSK images and measurements of the CSA of the SM. The researchers found techniques to be of high quality and reliable when looking at intra-rater values of changes in CSA. In agreement, Temes, et al. [32] assessed 15 asymptomatic individuals aged 30-49. They compared the measurements taken by three different evaluators and found the results of resistance of the SM caused significant changes in the CSA of the muscle. Finally, Muraki, et al. [33] observed elasticity of the SM and tendon looking at similar aspects of the muscle and found intra-rater measurement of CSA through ICC of 0.93-0.99 when observed under loaded conditions. For the four referenced studies referenced in this paragraph, the testing position in each of the other studies was in the EC or FC position. The present study used the DHA position. The results of the study on increased resistance causing increases in CSA using the DHA test were similar regardless of the varied testing protocols [16].
In the Forbush, et al. [16] study, the DHA test showed the largest CSA mean change when compared to EC and FC testing, though all three positions showed significant changes in CSA. The DHA position may reduce the ability of the deltoid to contribute to the contraction because of extreme position of horizontal adduction. Increased deltoid contribution to the FC and EC tests of the SM has been shown through other studies [34-36]. The middle deltoid muscle was found to be equally as active as the supraspinatus while performing the EC test by Lee, et al. [36]. In addition, the FC test also showed large amounts of middle deltoid muscle activity during testing. Given that isolation of the supraspinatus during clinical testing is reported as difficult, further testing of the deltoid contribution to the DHA test is still indicated [36-38].
Diagnostic musculoskeletal ultrasound is supported as a valid technique when compared to MRI and reliable in intra-rater values [17,19,24,39]. Intra-rater reliability has been demonstrated through several previous investigations to be consistently above 0.85. Each of these authors found inter-rater reliability that was less than intra-rater reliability but still were found to be near 0.70. Suggestion could be made that the measurements made in this study should be reliable through the use of one trained investigator (intra-rater).
In the Forbush, et al. [16] study, the resistance given to each subject was manually applied and was not measured through dynamometry. In the present study, resistance was measured through max loading and then a 50% level was calculated and maintained through contraction. No differences in CSA values of the SM were found between the original investigation and this study.
Limitations were revealed in this study. The group studied was a mostly homogeneous group with limitations in the range of ages. The same MSK tool was utilized by a lone researcher to capture images and record CSA. Some researchers feel this may bias the study, but this increases reliability through intra-rater readings. Future studies are planned to capture validity and reliability data for the DHA test with an active patient population and data collection in order to determine the most efficient method to isolate the SM in evaluative testing.
The cross-sectional measurement of the SM using MSK at the supraspinous notch in the superior shoulder region was consistent with the amount of force generated by the muscle during contraction. Greater force led to greater cross-sectional dimension. This change in measurement would suggest CSA can be used to objectify increased contraction of the supraspinatus in comparing strength of the SM when using the DHA test.
Cross-sectional area using MSK ultrasound might be able to be used by trained individuals to determine the activity and force produced by the SM of an individual without the need for further investment in EMG. Increases in CSA of the SM would relate to increases in force production by the SM. Musculoskeletal ultrasound should provide an alternative measure for evaluating the health and strength of the SM.