Background: Chronic fatigue syndrome (CFS) is a poorly understood disease and information on effective treatments is sparse. A vast variety of vague treatment regimens including self-treatment with over-the-counter painkillers and muscle relaxants with little definitive evidence in improving the course of disease has further augmented the complexity of management of CFS. The aim of this review article is to compare and contrast the efficacy of different treatment protocols currently in practice based on a four-category regimen system.
Methods: We performed a systematic search of 612 studies on PubMed and other sources and found 51 frequently prescribed medications for CFS. These treatments were classified into four categories I-IV (category I being the most effective therapies and category IV being the least).
Results: Out of 51 frequently prescribed medications for CFS, 3 (Omega 3 Fatty Acids, Nexavir, Amphotericin B) have demonstrated high response rates (50%+), high symptom remission (50%+) and high full remission rates (40%+).
Conclusion: Our understanding of CFS/ME is rapidly evolving. Numerous research discoveries have been made in the key aspects of CFS/ME, disease prevalence, etiology, biomarkers symptom manifestation, and treatment modalities. The four-category drug regimen outlined in our systematic review can be used as a starting point for future CFS/ME treatment and research.
Chronic fatigue syndrome, Treatment, Efficacy, Regimen
For nearly 100 years, [1] Chronic Fatigue Syndrome (CFS), also known as Myalgic Encephalomyelitis (ME) has puzzled doctors and patients alike. Patients fell ill and became severely disabled with a wide range of diffuse symptoms and for seemingly no reason with no chance of recovery.
Due to the lack of understanding of CFS and its diffuse clinical picture, there was not much research funding for CFS/ME until recently and 80% of CFS/ME patients are still undiagnosed and have not received proper treatment [2].
Fortunately, numerous research advancements have been made, which are being collectively called as "A reboot for Chronic Fatigue Syndrome research" [3] and have enabled researchers to gain $35 million in additional funding for further research from 2018 to 2023 across 4 different research hubs in the U.S.
At this point, comprehensive, 700-pages long summaries on CFS with hundreds of proposed treatments exist [4-6]. However, a quantitative evaluation on the effectiveness of those treatments does not exist.
For that reason, this review aims to show a complete overview of the existing literature and to distill the wide complexity disease that is still very hard to understand into the most relevant points of the disease.
This review study was conducted on a medical research database (PubMed) by searching the keyword "Chronic Fatigue Syndrome [Title]" for studies published within a designated time period from January 1st 2015 to October 1st, 2020 with free full text articles available. The initial cohort of papers was screened using the inclusion criteria: (a) studies conducted in the aforementioned timeframe, (b) case reports, case series, observational studies, population-based studies, randomized control trials, meta-analysis related to the aforementioned keywords, and full-text studies in English language.
The PubMed search results returned 571 studies. An additional 41 studies from various other sources meeting the inclusion criteria regardless of publishing date were also considered. A total of 612 studies were screened of which 509 were excluded as they did not meet the inclusion criteria. After assessing the rest of 103 full-text articles for eligibility, 45 studies were further excluded due to duplicate findings and due to their lack of clinical relevance. A total of 58 studies were included in the final analysis. PRISMA guidelines were followed through the review process and figure 1 describes the data analysis process in detail (Figure 1).
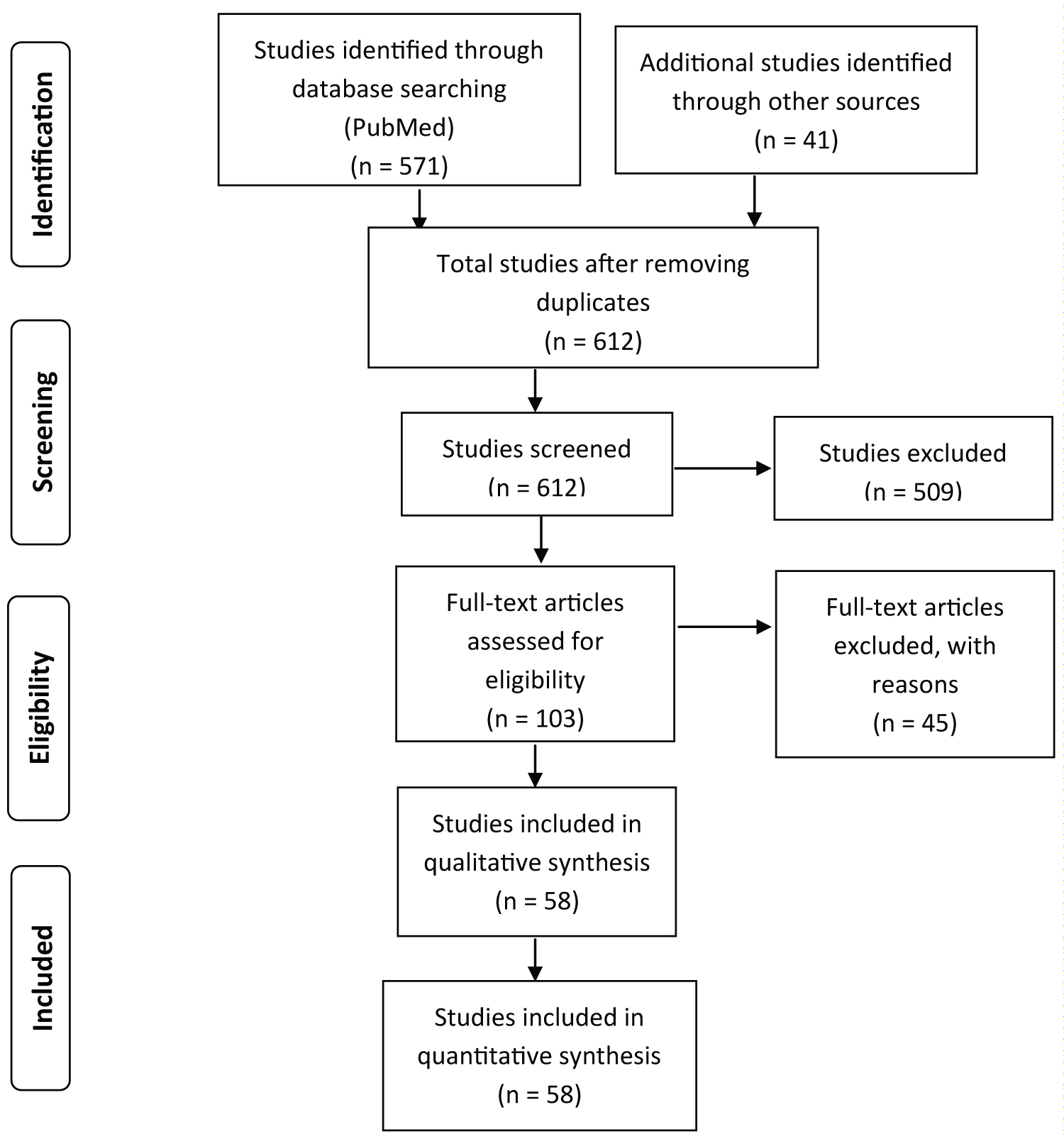 Figure 1: PRISMA diagram for systematic analysis of PubMed articles on CFS and articles from other sources.
View Figure 1
Figure 1: PRISMA diagram for systematic analysis of PubMed articles on CFS and articles from other sources.
View Figure 1
The prevalence of CFS/ME is significantly high, with an estimated 0.4% among the general population and 1% in children [7]. A large gender gap exists, similar to autoimmune diseases with middle-aged women comprising 70% of CFS patients; women are twice as likely to have CFS compared to men. CFS is found in people of all ages and races. A case as young as 4-years-old has been reported. Additionally, it is more common in adolescents than in children. Cases can occur in clusters or sporadically [8]. In children, a 2011 study found that among 465 of 2855 school children that were absent from school, 28 (1.0%) had CFS, diagnosed on the basis of Fukuda criteria. Upon follow up, out of the 19 children, 6 had fully recovered at 6 weeks and a further 6 at 6 months. A total of two-third of children recovered from CFS within 6 months [9]. Other studies from 2016 and 2018 confirmed the incidence of CFS with 0.9% among middle school children and with 2.4% among adolescents, respectively [10,11].
Due to the high prevalence and severe disability, CFS is estimated to cost the U.S. economy $17 to $24 billion annually in medical bills and lost incomes [12].
The incidence of the disease at the end of the 20th century was estimated at 2-7 cases per 100,000 population. At the the beginning of the 21st century, this number rose to 30-50 cases per 100,000 population. The reason for rise in incidence is twofold; an improvement in the awareness and diagnostic modalities, and an actual rise in the number of people suffering from CFS/ME [13].
Recent findings have identified various causes of CFS/ME, with the common denominator being heavy stress on the body and/or the immune system causing a wide range of mitochondrial dysfunction [14-21].
1. Viral infections, such as Influenza and herpes, infections caused by EBV, CMV, Varicella, HHV6, and HHV7
2. Bacterial infections, such as pneumonia
3. Blood transfusions
4. Physical injuries such as trauma and burn injuries
5. Yeast infections
6. Susceptible immune system
7. Hormonal imbalance
8. Mold exposure
9. Heavy metal exposure
10. Vaccinations
11. Mental health problems, e.g. mental illness, stress, and emotional trauma
12. Genetic predisposition - CFS/ME is more common in blood relations
There are around 50 symptoms that are commonly reported in CFS patients [22,23]. With 97.9% of patients experiencing post-exertional malaise, it is the most common symptom of CFS. Other common symptoms include the following, sorted from most commonly experienced to less commonly experienced:
1. Fatigue, often accompanied by non-restorative sleep, generally worsened by exertion (Post-exertional Malaise): 95-100%
2. Muscle weakness: 85-95%
3. Low blood pressure: 86%
4. Headache: 75-95% (daily headache: 50%)
5. Malaise: 80%
6. Cognitive symptoms including confusion, lightheadedness, inability to think clearly, concentration/attention deficit, depression, mood swings, excessive irritability, overreaction, memory problems (especially short-term memory), anxiety: 65%-100%
7. Muscle and/or joint pain, neck pain: 65-95%
8. Fevers/chills/sweats/hot flashes: 60-95%
9. Heat/cold intolerance: 75-80%
10. Aphasia and/or dyscalculia: 75-80%
11. Sleep disorder/disturbance (insomnia, non-restorative sleep, unusual nightmares): 65-100%
12. Photosensitivity: 65-90%
13. Disequilibrium, spatial disorientation, dizziness, vertigo: 60-90%
14. Nausea: 60-90%
15. Irritable bowel syndrome (diarrhea, nausea, gas, abdominal pain): 50-90%
16. Chronic sore throat: 50-90%
17. Recurrent illness and infections: 70-85%
18. Seizure-like episodes: 70% (seizures: 2%)
19. Fungal infection of skin and nails: 71%
20. Increased/severe PMS: 70%
21. Muscle twitching, involuntary movements: 55-80%
22. Personality change: 55-75%
23. Painful and/or swollen lymph nodes: 50-80%
24. Subnormal body temperature: 65%
25. Swelling, fluid retention: 55-70%
There are 28 more symptoms with an average 60% incidence or less recorded: Alcohol intolerance: 45-75%, coordination problems/clumsiness: 60%, weight gain: 50-70%, bladder/prostate problems, frequent urination: 20-95%, , shortness of breath: 30-70%, difficulty swallowing: 55-60%, sinus pain 56%, systemic yeast/fungal infections: 30-80%, heart palpitations: 40-60%, severe allergies: 40-60% cfs, sensitivities to medicines, inhalants, odors, and foods: 25-65% rash or flushing of face: 35-45%, visual disturbance (scratchiness, blurring of vision, "floaters"): 45-55%, episodic hyperventilation: 40-45%, fainting or blackouts: 40%, chest pain: 40%, panic attacks: 30-40%,, eye pain: 30%, pressure at the base of the skull: 30%, hair loss: 20-35%, weight loss: 20-30%, tendency to bruise easily: 25%, vomiting: 20%, paresthesia (numbness, tingling or other odd sensations in face and/or extremities): 25-60%, strange taste in mouth (bitter, metallic): 25%, temporary paralysis after sleeping: 20%, earache: 20%.
Since patients often experience many of these symptoms together, they are often severely disabled for many years due to the inability to perform routine physical activities, such as housekeeping or grocery shopping. This can lead to further complications such as unemployment (50%), social maladjustment, a lowered quality of life and side effects of drugs associated with long term use of painkillers [24].
Until recently, no biomarkers with reliable specificity had been identified for CFS/ME. However, in 2015, a study performed in Germany, found that 29% of CFS/ME patients have elevated autoantibodies to M3 and M4 muscarinic acetylcholine receptors [25]. In 2017, 17 cytokines were found to be associated with CFS severity. Furthermore, thirteen of these 17 cytokines are primarily pro-inflammatory: CCL11, CXCL1, CXCL10, IFN-γ, IL-4, IL-5, IL-7, IL-12, IL-13, IL-17, leptin, G-CSF, and GM-CSF. Interestingly, 11 of the 17 cytokines and 9 of the 13 pro-inflammatory cytokines share a similar structure, i.e., a four α-helical bundle structure. Their linear relationship with severity was statistically significant, though the study did not adequately distinguish cases from controls. This apparent paradox is explained by the levels of these cytokines in patients with mild disease being in the lower segment of the normal range for healthy controls and the levels in patients with severe disease being in the upper segment of the normal range for healthy controls [26]. Compared to controls, sustained increase in plasma TNF-α after exercise in CFS/ME patients compared to controls has been observed [27]. Moderate exercise has also been reported to induce a larger 48-hour post-exercise area under the curve for IL-10 [28].
In 2017, the first specific biomarker was reported to be Activin B [29]. In 2019, the second specific biomarker for CFS/ME via nano-electronic blood based analysis was revealed [30]. More research findings and treatments emerged over the last few years, with a further $35 million funding being committed to CFS research from 2018 to 2023 across 4 different research hubs in the U.S. The latest developments have been described as "A reboot for Chronic Fatigue Syndrome research" [3]. A multitude of non-specific biomarkers have been established along with 2 specific biomarkers for CFS/ME [31].
Since 60% of patients do not recover and the recovery in the other 40% of patients is slow, finding effective treatment is paramount [32]. More than 300 different pharmaceuticals and supplements have been considered and trialed by doctors for CFS, and the vast majority of these turned out to have little or no efficacy [4-6].
We evaluated the efficacy of 56 treatments for CFS for which data from trials existed in terms of demonstrated response rate, remission of some symptoms, complete remission of CFS, time to achieve complete remission, and strength of available evidence. Based on these five parameters, four categories (I-IV) of treatment regimen are proposed for devising a prompt and effective management plan. After thorough analysis, six were able to provide Category I efficacy, five Category II efficacy, six Category III efficacy, and 35 medications were included in the Category IV.
Levels of evidence were defined as per the Oxford Criteria for levels of evidence (Figure 2).
 Figure 2: Oxford criteria of levels of evidence.
View Figure 2
Figure 2: Oxford criteria of levels of evidence.
View Figure 2
Category I treatment regimen:
The following criteria were adopted for the inclusion of drugs in Category I:
1. Overall response rate
2. Remission rate of CFS symptoms
3. % Responders with 100% remission
4. Time to achieve complete 100% remission/ some improvement
5. Level of evidence (Defined as per the Oxford Criteria fig 2)
There are no FDA approved medications for treatment. Due to the wide range of symptoms of CFS, symptomatic treatment approaches vary but we suggest using a set of criteria based on best practices and evidence-based medicine. The table below shows 5 medications that meet the inclusion criteria for Category I treatment (Table 1).
Table 1: Category I medications with response rate, remission, time to remission and level of evidence. View Table 1
Category II treatment regimen:
The following criteria were adopted for the inclusion of drugs in Category II:
1. More than 50% average response rate
2. More than 50% remission rates of CFS symptoms
3. No documentation on % responders with 100% remission
4. Time to achieve symptom improvement between 3 to 6 months
5. Level I or II evidence
Table 2 below shows 7 medications that meet the inclusion criteria for Category II treatment.
Table 2: Category II medications with response rate, remission, time to remission and level of evidence. View Table 2
Significant remission in symptoms is denoted in a 50% remission percentage. Furthermore, not all studies investigating the above treatments were conducted in a large-scale and double-blinded setting.
Category III treatment regimen:
The following criteria were adopted for the inclusion of drugs in Category III:
1. More than 50% response rate
2. Less than/Equal to 50% remission rate of CFS symptoms
3. No documentation of % responders with 100% remission
4. Time to improvement between 3 and 6 months
5. Level II evidence or higher
Table 3 shows 5 medications that meet the inclusion criteria for Category III treatment;
Table 3: Category III medications with response rate, remission, time to remission and level of evidence. View Table 3
Category IV treatment regimen:
The following criteria were adopted for the inclusion of drugs in Category IV:
1. No documentation of patient response rate
2. No documentation of remission rate of CFS symptoms
3. No documentation of % responders with 100% remission
4. No documentation of time to improvement between 3 and 6 months
5. Level IV or V evidence
A total of 31 supplements fall in this category. These include Sodium bicarbonate/baking soda, Ibudilast, Cordyceps Sinensis and Shiitake Mushroom, Oxytocin, Larorice, Biotin, Curcumin, Inosine, Epicor, Milk Thistle, Colostrum, NAC, Propionic Acid, Microbiotics, Hawthorns, L-Glutathione, Organic Germanium, Artesunate, IP-6, Spinola, Sulforaphane, L-Serine, Moringa Oleifera, Quercetin, NeuroProtek, CoEnzyme Q10 + NADH, St. John's, GcMAF, and Melatonin. Additionally, there are 6 pharmaceutical formulations (Naphazoline, Piracetam, Pentoxifylline, Azithromycin, Guaifenesin, Fludrocortisone) that fulfill the inclusion criteria for Category IV treatment regimen. However, the effectiveness of these supplements and pharmaceuticals has not been published or investigated in scientific studies and their evidence is hypothetical, informal, or based on expert opinion of medical professionals only.
Behavioral treatment or cognitive behavioral therapy (CBT) has been widely used for CFS until recently. However, surveys from patient organizations have found considerable rates of harm from CBT in CFS patients [50,51]. Moreover, the two largest UK patient charities, ME Association and Action for ME, have called for the use of CBT to challenge "illness beliefs" to be withdrawn and for health care providers to warn patients of the potential for harm [52]. What's more, the largest independent patient survey of patient experiences of CBT and GET in the UK found that ME/CFS patients were almost double as likely to have their mental health deteriorate than improve as a result of the course and see new symptoms develop and a negative effect on their physical health [53].
Recovery from CFS is more common than assumed with 40% of CFS patients reporting symptom improvement after several years [54,55].
Longitudinal studies have shown that 17-64% of CFS patients with longer than 6 months of illness improved, less than 10% fully recovered, and another 10-20% worsens during follow-up. Older age, longer duration of symptoms, severity, comorbid psychiatric illness, and poor physical health status are additional factors that worsen the prognosis. Children and adolescents appear to recover more rapidly and have a low incidence of recurrence [56].
Recovery of CFS with long term follow-up of 784 pediatric CFS patients was demonstrated in a study performed in Australia. 35% of patients recovered at 5 years and 68% at 10 years post diagnosis, with 5% remaining very unwell and 20% significantly unwell. However, the study failed to identify predictors for recovery [57].
A review of 14 studies found that on average 5% of patients recovered (range 0%-31%); 40% of patients improved during follow-up (range 8%-63%); 8%-30% returned to work; 5%-20% of patients reported worsening of symptoms. Furthermore, prompt diagnosis and early treatment significantly raises the rate and pace of recovery [54,58].
CFS/ME is not a mysterious disease anymore, but a disease with known prevalence (0.4%-1%), etiology (12 different causes), biomarkers (2 specific and 17 unspecific), symptom manifestation (50) confirmed by multiple studies. The evidence suggests the 10 different agents as the most effective treatments for CFS.
Furthermore, the evidence suggests the following 10 different agents as the most effective treatments for CFS.
Category I treatment options with the highest response rates (50%+), high levels of evidence and highest full remissions rates:
1. Omega-3 Fatty acids
2. Nexavir
3. Amphotericin B
Category II treatment options with the high response rates (50%+) and high levels of evidence, but no documented full remission rates were:
1. Magnesium injections
2. Vitamin B12
3. Thymic protein A
4. Equilibrant
5. Heparin injections
6. Acetyl-L-Carnitine
7. Amino Acid Complex
There are certain limitations to this review. Around 40% of CFS patients experience recovery over several years regardless of treatment. Thus, 40% of patients receiving the above treatments will see slight improvement regardless. However, most of the above studies were conducted over the time span of a few weeks to 6 months, which should alleviate this limitation.
Moreover, most of the above studies were not independent studies with committee oversight, which can also skew data.
As well, due to the complex nature of CFS, it has varying presentations and a general heterogeneity in etiology. Owing to this, some treatments that might work well for a specific group of CFS patients might not work well for others.
For that reason, future research is required to define the exact classification of subtypes while diagnosing CFS patients for future trials. This is not important only for trials that investigate the effectiveness of treatment, but also for all other medical trials investigating the underlying mechanisms of CFS/ME.
With the number of studies with the title "Chronic Fatigue Syndrome" from 13 studies published on PubMed in the year 2000 study to 121 studies published in 2019, having increased by nine times and significant amounts of newly committed funding, the outlook for future research and better treatments also looks promising.
Research in CFS/ME treatment has come a long way. Yet, there is no standard scientifically demonstrated evidence-based intervention in practice. This overview has compared the frequently prescribed medications for CFS from available research studies and classified them into four categories in terms of clinical effectiveness.
The evidence suggests that Category III treatments only provide symptomatic relief and can only reduce cognitive symptoms as a consequence while Category I and Category II treatments are causal treatments and are thus able to reduce all symptoms.
With this analysis, CFS patients are now able to make use of a treatment regimen based on the available evidence, which suggests that potentially several million CFS patients could achieve full recovery with the Category I treatments.
The authors declare they have no conflict of interest.