Sciatic nerve entrapment due to fibrous adhesion (SNE-A) in the deep gluteal space is an under diagnosed entity that is responsible for a portion of low back pain (LBP) cases. This case series aimed to (1) Propose new clinical diagnostic criteria, (2) Determine the frequency of SNE-A among a population with LBP, and (3) Assess clinical outcomes after manual therapy for SNE-A.
De-identified patient records were obtained from four United States-based clinics. All patients presenting with a chief complaint of LBP between February and July 2019 were retrospectively reviewed. Patient records were evaluated for a primary diagnosis of SNE-A using the proposed diagnostic criteria: (1) Straight leg raise (SLR) range of motion of 75° or less; (2) SLR-produced symptoms; (3) Addition of dorsiflexion at the end of an SLR increased symptom intensity and/or increased symptom area; and (4) Palpation of sciatic nerve mobility in the deep gluteal space revealed decreased motion. Those patients diagnosed with SNE-A received manual therapy, and clinical outcomes were assessed, including SLR range of motion, Numeric Pain Rating Scale (NPRS) scores, and patient estimated percent improvement.
SNE-A was diagnosed as being primarily responsible for symptoms in 13 of 132 (9.8%) patients with LBP. Each patient was treated with manual therapy designed to reduce fibrous adhesion (Manual Adhesion Release®). Treatment resulted in a SLR range of motion improvement of 20.8° ± 9.2°, an NPRS improvement of 3.7 ± 1.3 points (64% improvement), and a patient estimated percent improvement of 67.3 ± 25.4%. In this series, those patients with SNE-A constituted around a tenth of patients with LBP.
New clinical diagnostic criteria for SNE-A can be applied to identify a group of patients with LBP that could be expected to respond well to manual therapy, though prospective controlled studies are warranted.
Fibrous adhesion, Low back pain, Manual adhesion release, Piriformis syndrome, Straight leg raise
EPI: Estimated percent improvement; LBP: Low back pain; NPRS: Numeric pain rating scale; SLR: Straight leg raise; SNE-A: Sciatic nerve entrapment due to fibrous adhesion
Low back pain (LBP) is the second most common reason for a primary care visit, with total associated costs exceeding 100 billion dollars annually in the United States [1,2]. Low back pain is not a diagnosis, but rather a symptom for which a specific diagnosis should be established before starting treatment [3]. Entrapment of the sciatic nerve should be included in the differential diagnosis of LBP [4,5]; among several sites where entrapment may occur, the sciatic nerve commonly becomes entrapped in the deep gluteal space via fibrous bands (Figure 1) [6-8]. Endoscopic visualization typically reveals limited motion of the sciatic nerve and diminished epineural blood flow secondary to the fibrous adhesion [6,9]. Surgical reduction of adhesions has been demonstrated to restore mobility and blood flow while reducing pain and improving function [6,7], but it is recommended only when conservative treatments have failed [9,10]. However, conservative care for sciatic nerve entrapment due to fibrous adhesion (SNE-A) has not been well defined or adequately studied [11].
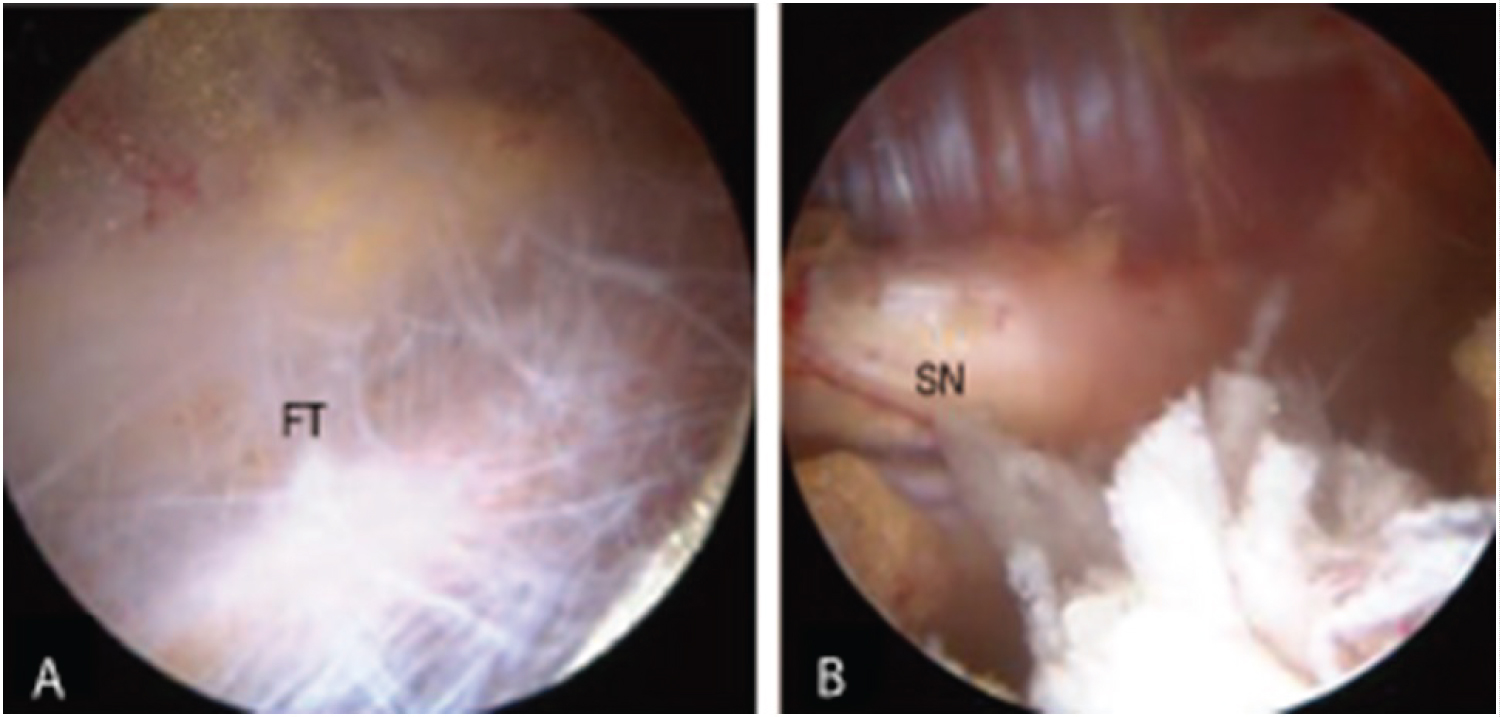 Figure 1: Endoscopic images of (A) Fibrous tissue (FT) surrounding the sciatic nerve in the deep gluteal space and (B) The sciatic nerve (SN) following adhesion reduction. Modified from Ham, et al. [8] (https://doi.org/10.5371/hp.2018.30.1.29) and licensed under Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0; https://creativecommons.org/licenses/by-nc/4.0/). The original figure has been modified to show only two of the eight images.
View Figure 1
Figure 1: Endoscopic images of (A) Fibrous tissue (FT) surrounding the sciatic nerve in the deep gluteal space and (B) The sciatic nerve (SN) following adhesion reduction. Modified from Ham, et al. [8] (https://doi.org/10.5371/hp.2018.30.1.29) and licensed under Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0; https://creativecommons.org/licenses/by-nc/4.0/). The original figure has been modified to show only two of the eight images.
View Figure 1
Existing literature suggests that a sciatic nerve entrapment diagnosis can be established based on a patient's medical history, physical exam, and imaging data [12]. Here, the author proposes new clinical diagnostic criteria that may improve early identification of this under-recognized diagnosis. Furthermore, as there is a need for well-defined conservative care, the author reports treatment results of a manual therapy (Manual Adhesion Release®) specifically designed to reduce adhesion between the sciatic nerve and surrounding structures in the deep gluteal space.
The aims of this study were to: (1) Propose new clinical diagnostic criteria for SNE-A, (2) Establish the frequency of SNE-A in an LBP population, and 3) Determine clinical outcomes following manual therapy (Manual Adhesion Release®).
This retrospective case series included patient data from four United States-based ambulatory conservative care clinics. Diagnosis and treatment were performed by licensed Doctor of Chiropractic practitioners that were selected based on their duration of training (minimum of 200 hours each) and their clinical experience (mean experience of 9 ± 3 years) with the studied diagnostic (Integrative Diagnosis Lumbar and Hip Course) and treatment methods (Manual Adhesion Release®). File reviews were performed to ensure consistent clinical data collection.
All consecutive patient files listing an initial examination date from February to July 2019 and a chief complaint of LBP were obtained by the author for analysis (Figure 2). Data collection was completed by November 2019. The files were de-identified by the treating practitioner prior to providing the author access to the records.
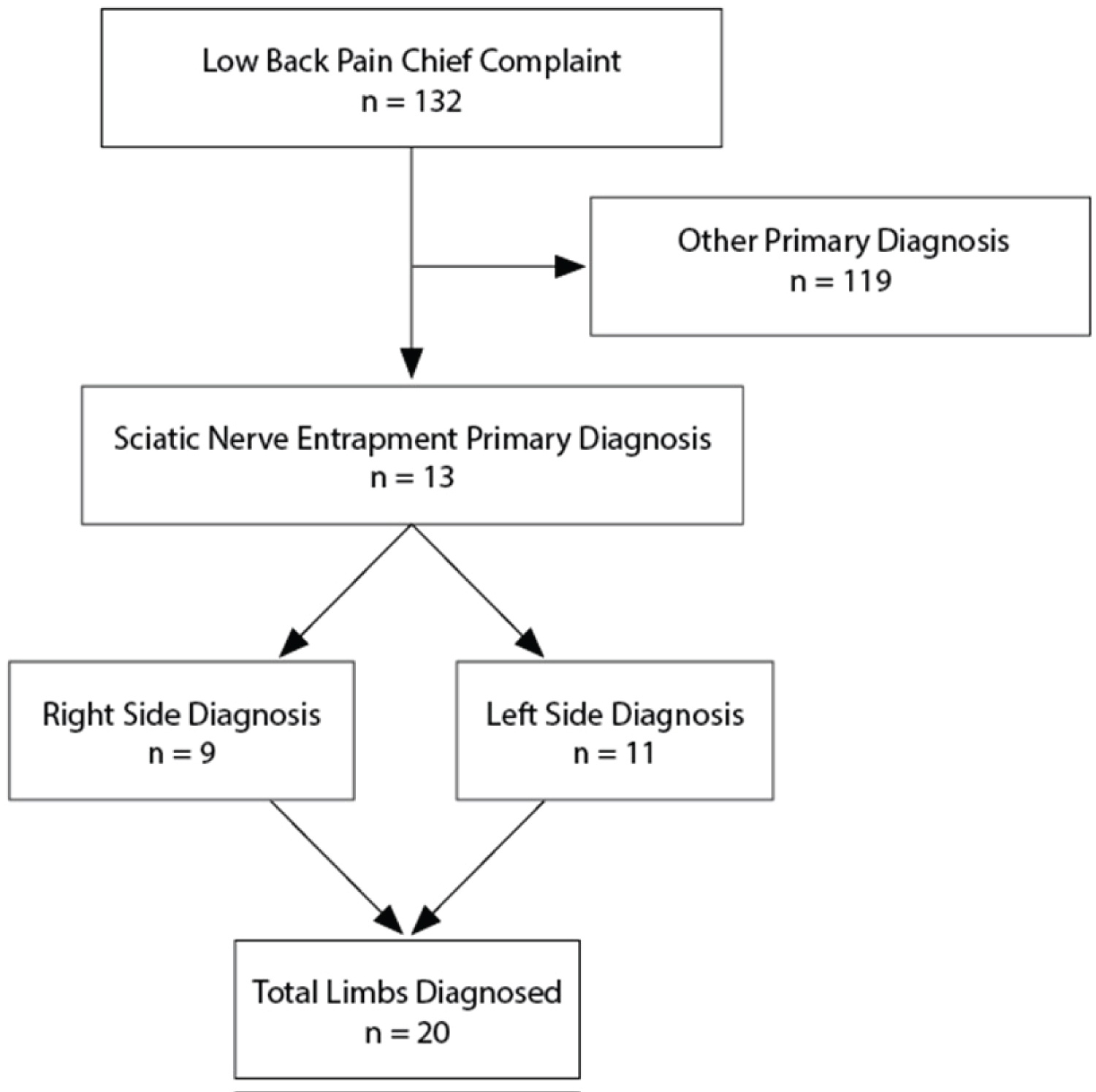 Figure 2: Flowchart of the study design.
View Figure 2
Figure 2: Flowchart of the study design.
View Figure 2
Low back pain is defined as pain, muscle tension, or stiffness that is localized below the costal margin and above the inferior gluteal folds, with or without posterior thigh symptoms [13].
The LBP cases were further analyzed to confirm the primary diagnosis of SNE-A using the diagnostic criteria developed by the author. These inclusion criteria consisted of: (1) A straight leg raise (SLR) range of motion of 75° or less; (2) SLR producing symptoms of pain, tension, or stiffness (not required to reproduce chief complaint); (3) Addition of dorsiflexion at the end of the SLR resulting in increased symptom intensity and/or enlargement of the area where symptoms were reported; and (4) Palpation of sciatic nerve mobility with application of a lateral to medial bowing force perpendicular to the nerve in the deep gluteal space revealing decreased motion and increased tension.
Exclusion criteria included the following: (1) Other diagnosis was determined to be responsible for more than 50% of the items assessed in the clinical presentation (lumbar disc pathology, hip joint pathology, other nerve entrapment, etc.) and/or (2) A greater percentage of lumbar flexion, hip flexion, or hip extension range of motion compared to that of the SLR. Other diagnoses were established by standard means based on patient history, physical exam, palpation, and imaging when necessary and available. For the range of motion exclusion criteria, all ranges of motion listed above were measured, converted into a percentage of the total range, and ranked highest to lowest. Cases in which the SLR did not exhibit the most restricted range of motion were excluded.
The SLR was performed with the patient lying supine with the spine in a neutral position. A hand-held inclinometer was placed on the mid-tibia and the examiner moved the patient into hip flexion, maintaining knee extension and monitoring for compensatory movements. This is a valid method for measuring limb elevation angle [14]. The motion was continued until each patient reached the limit of symptom tolerance, or moderate resistance or compensation occurred. Holding this position, the range of motion was recorded, symptom location was noted, and gentle dorsiflexion was applied. Any symptom changes upon dorsiflexion were then recorded. This is an appropriate sensitizing maneuver to assist structural differentiation between neural tension and hamstring restriction [15].
Locating and palpating the sciatic nerve for mobility testing was performed following the following steps:
1. The patient was asked to lie on their side with the affected side facing up. The hip was flexed to 45° and the knee was flexed to 90° with slight hip adduction (resting on the exam table).
2. The practitioner then identified the lateral portion of the greater trochanter, then the posterior portion of the greater trochanter, followed by palpation 2-5 cm medial to this landmark, identifying the lateral edge of the sciatic nerve deep to the gluteus maximus.
3. From this location, a lateral to medial force was applied perpendicular to and against the sciatic nerve. Movement of less than 1 cm with increased resistance and tension is a palpatory indicator of the presence of fibrous adhesion.
4. Palpation continued along the sciatic nerve, superior to the piriformis and inferior to the quadratus femoris. Areas of decreased mobility were identified as the location of the adhesion.
All patients that met the diagnostic inclusion/exclusion criteria for SNE-A were treated by Manual Adhesion Release® for the sciatic nerve at the posterior hip. The treatment protocol consisted of the following steps:
1. From the palpation position, the provider made reinforced thumb contact directly against the patient's skin, distal to and in contact with the fibrous adhesion.
2. An assistant extended the patient's knee to set optimal tension on the adhesion (taking the "slack" out of the nerve and muscles).
3. The provider applied a force perpendicular to the external rotator muscles, sufficient to achieve a contact depth equivalent to the depth of the sciatic nerve.
4. Maintaining this depth and force, the practitioner shifted his/her position and weight to apply a force parallel to the sciatic nerve, stretching and applying tension to the adhesion.
5. The assistant moved the patient into hip flexion, maintaining knee extension, while the practitioner maintained depth and tension against the adhesion.
6. This produces a tension force on the adhesion and encourages motion between the sciatic nerve and adjacent tissues. Multiple passes were performed (usually 3-5) during each treatment session.
The numeric pain rating scale (NPRS) was used to assess pain at the time of intake and at the end of SNE-A treatment. Patient estimated percent improvement (EPI) was determined at the start of each visit.
The rate of SNE-A diagnoses among patients with LBP was determined by a standard percentage calculation. Paired sample t-tests were used to assess changes between the pre- and post-treatment NPRS scores and SLR ranges of motion. A value of P < 0.05 was considered statistically significant. Statistical analysis was conducted using MedCalc software version 19.3.1 (MedCalc Ltd., Ostend, Belgium). Values are expressed as mean ± standard deviation (SD).
In total, 132 LBP cases were analyzed. Of these, 13 patients (9.8%; male: n = 11, female: n = 2) were identified as having SNE-A as their primary diagnosis. The mean age was 41.4 ± 12.7 years. The left and right sides were affected in 11 and 9 cases, respectively, totaling 20 affected limbs. The average duration of symptoms that had been experienced at the time of the exam was 24.6 ± 40.1 months. Eleven of the 13 cases (85%) experienced prior treatment failure (Table 1).
Table 1: Clinical data of patients diagnosed with SNE-A. View Table 1
The pre-treatment SLR range of motion was 62.5° ± 6.9° and the post-treatment SLR was 83.3° ± 7.8° (t (19) = 10.1, p < 0.001), resulting in an improvement of 20.8° ± 9.2° (Figure 3). The pre-treatment NPRS score was 5.8 ± 1.4 and the post-treatment NPRS score was 2.1 ± 1.4, resulting in an improvement of 3.7 ± 1.3 (t (12) = -10.5, p < 0.001) or a 64% median improvement (Figure 4). Patient reported EPI was 67.3% ± 25.4 (Figure 5). The number of treatments required to reach maximum improvement was 3.7 ± 1.7. No complications or adverse reactions were recorded.
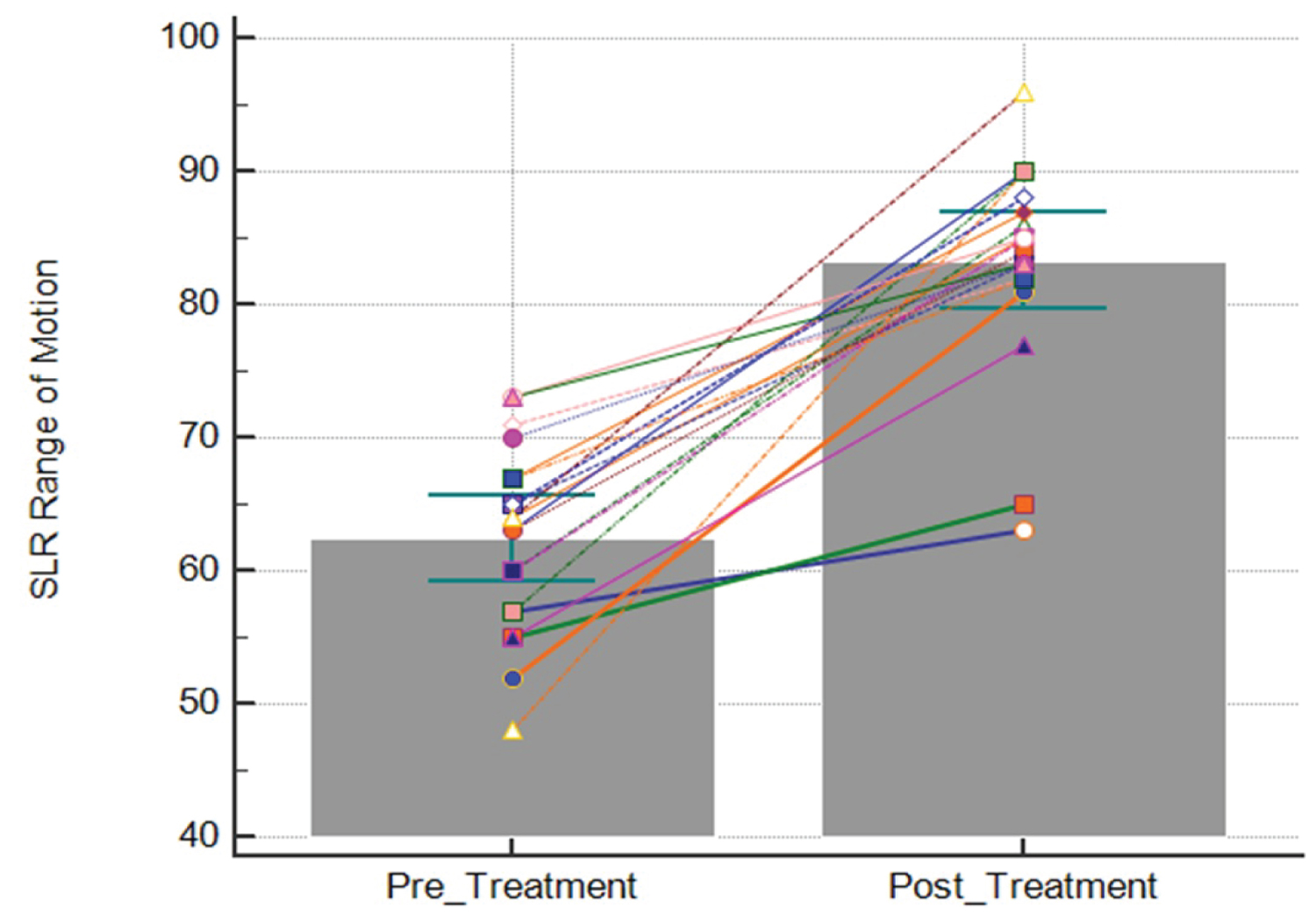 Figure 3: Straight leg raise (SLR) range of motion pre- and post-treatment. Each line represents the change in SLR range of motion (°) for each limb treated with Manual Adhesion Release®. Bars represent the mean. Error bars represent the 95% confidence interval.
View Figure 3
Figure 3: Straight leg raise (SLR) range of motion pre- and post-treatment. Each line represents the change in SLR range of motion (°) for each limb treated with Manual Adhesion Release®. Bars represent the mean. Error bars represent the 95% confidence interval.
View Figure 3
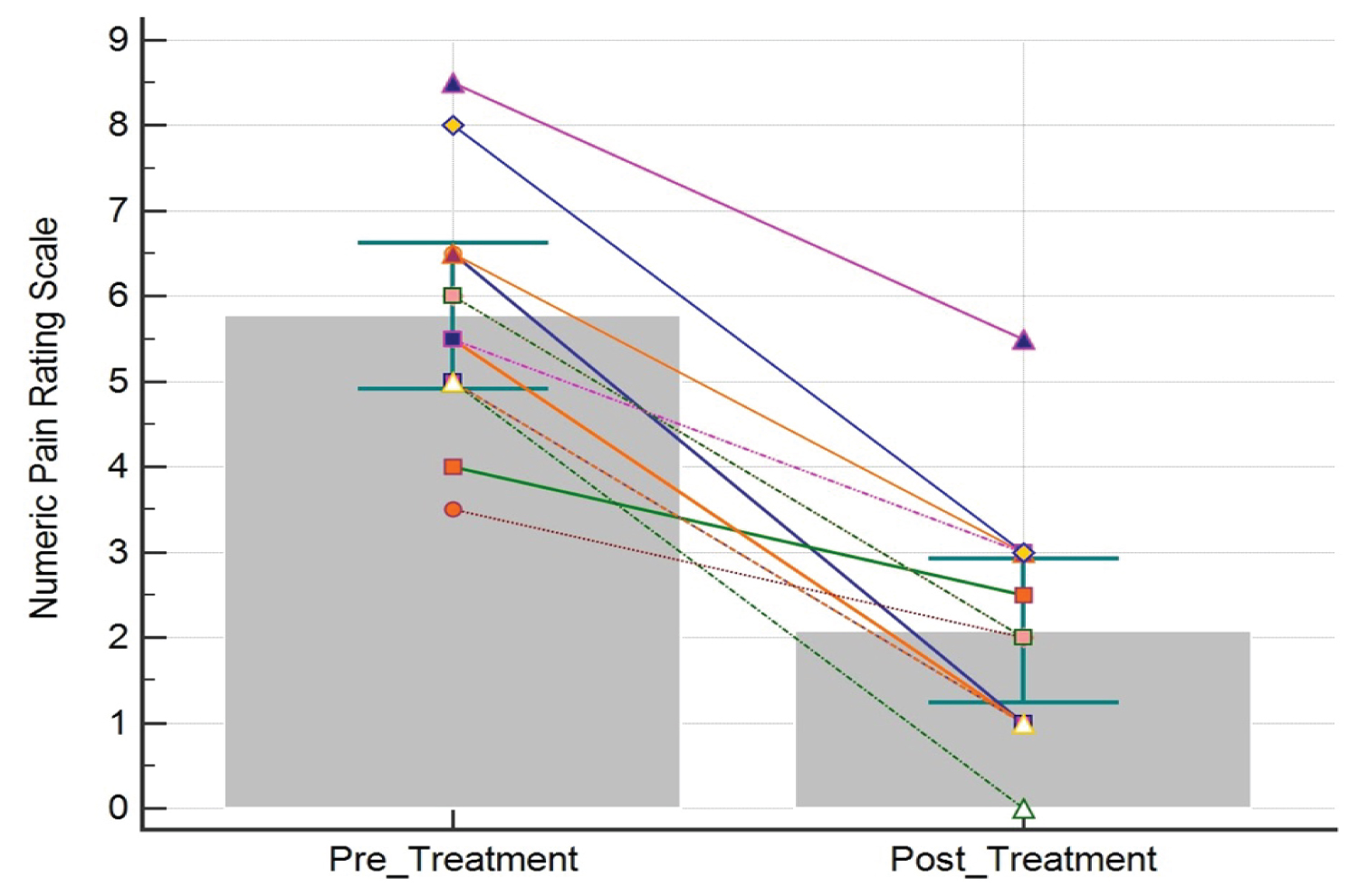 Figure 4: Numeric pain rating scale (NPRS) scores pre- and post-treatment. Each line represents the change in NPRS score for one patient. Bars represent the mean. Error bars represent the 95% confidence interval.
View Figure 4
Figure 4: Numeric pain rating scale (NPRS) scores pre- and post-treatment. Each line represents the change in NPRS score for one patient. Bars represent the mean. Error bars represent the 95% confidence interval.
View Figure 4
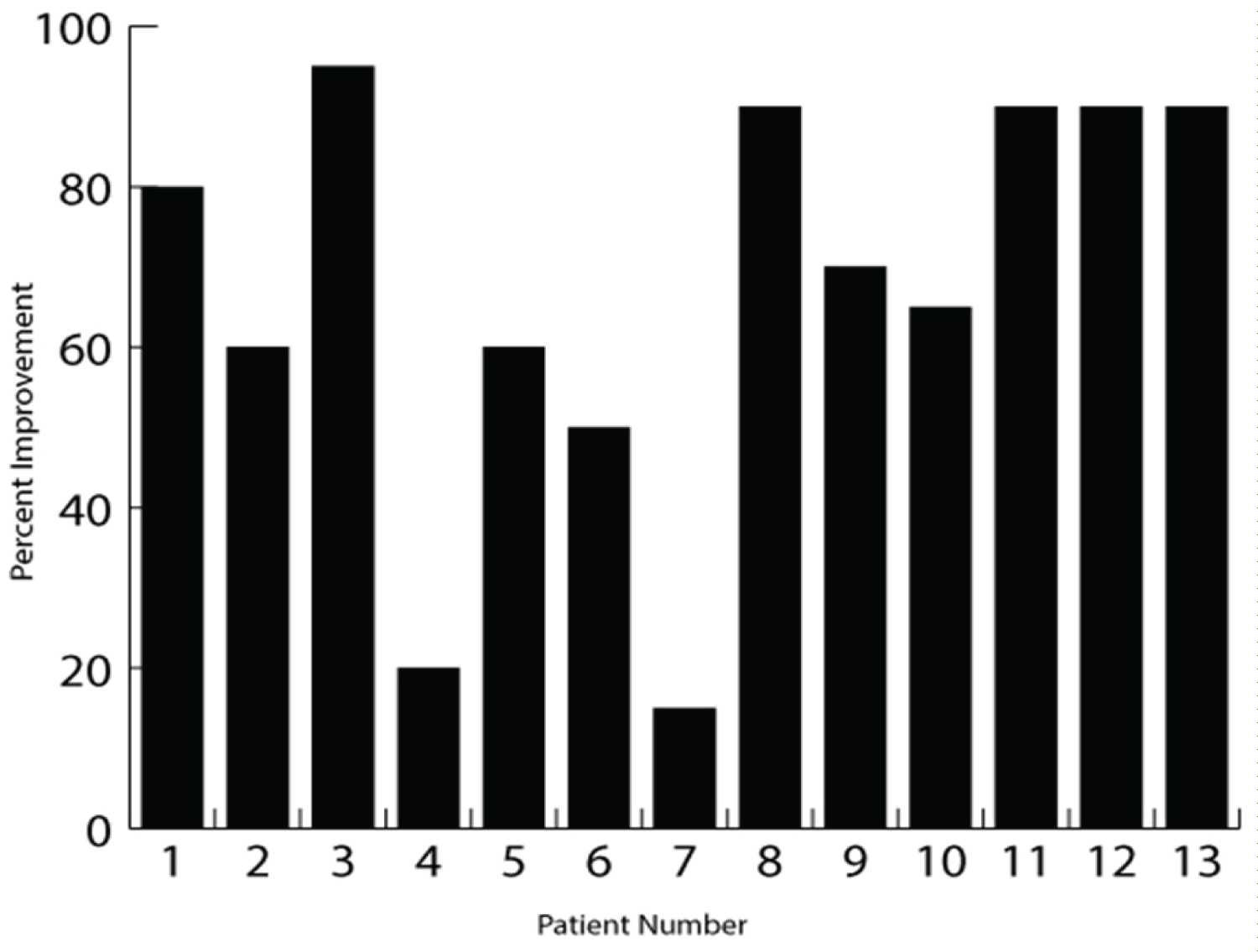 Figure 5: Patient estimated percent improvement (EPI; %). The figure shows the patient EPI at the completion of care for each patient.
View Figure 5
Figure 5: Patient estimated percent improvement (EPI; %). The figure shows the patient EPI at the completion of care for each patient.
View Figure 5
Among the patients with LBP, 9.8% had a primary diagnosis of SNE-A, which is similar to the previously reported rates of 6% and 17% for those experiencing symptoms arising from deep gluteal structures [16,17]. Only a general comparison can be made to these past studies, however, as those authors studied piriformis syndrome rather than the more specific SNE-A diagnosis assessed here.
Many treatments have been studied to improve SLR range of motion. Two studies of patients with LBP have reported mean SLR range of motion improvements of 11° and 12.4° [18,19]. Most studies have included healthy subjects and have assessed tight hamstrings and treatment utilizing various methods, including static stretching, neuromuscular facilitation, mobilization, and neurodynamic sliding techniques. These studies reported improvements in SLR range of motion of 4.7°-17.6°, with a mean improvement of 9.3° [20-25]. A meta-analysis concluded that neurodynamic treatment is superior to other treatments and no treatment at all in improving SLR range of motion [26]. The results of the present study demonstrated a two-fold greater improvement in SLR range of motion than the 9.3° (18%) mean previously reported, and the results exceeded the greatest range of motion improvement in the literature. This suggests there is a benefit to accurately identifying SNE-A as a primary diagnosis for LBP and that targeted treatment of these cases results in superior outcomes compared to non-specific treatments.
The author hypothesizes that the improved SLR range and decreased NPRS scores observed in this study are due to the deliberate application of tension to the exact location of the fibrous adhesion. In vitro research has shown that for extracellular collagen, tension loads of approximately 43 kPa (0.44 kg/cm2) and strain lengthening of 50-60% will cause tissue failure [27]. In contrast, manually produced compression and shear loads are unlikely to deform dense collagen fibers [28]. Therefore, manual therapy that does not generate the necessary tension, strain, and subsequent reduction of the fibrous adhesion is unlikely to be successful in reducing this pathology. Manual Adhesion Release® maximizes tension by locating the fibrous adhesion, administering manual contact adjacent to it, pre-positioning the patient so the target tissues are placed under moderate tension, adding parallel contact motion until the tissue is fully stretched, then adding patient motion to further increase tissue tension, while modifying the contact during such motion to maintain the vector of maximum tension. This is most effectively performed with direct skin contact while maintaining appropriate tissue depth.
Performing the SLR motion causes excursion of the sciatic nerve of up to 28 mm, with a strain of approximately 6% [29], yet the SLR test only has a reported sensitivity of 0.15 and a specificity of 0.95 in identifying individuals with sciatic nerve entrapment [30]. This low sensitivity is possibly due to traditional reliance on recreation of the chief complaint or the manifestation of a familiar symptom to qualify as a positive SLR test. On the other hand, more objective measurements are possible by assessing range of motion, and this study primarily used SLR as a test to measure range of motion. It has been shown that even a small amount of involuntary hamstring contraction reduces SLR range of motion [31], and this hamstring contraction may be a protective mechanism preventing excessive load on the sciatic nerve during the test [31]. In turn, this may result in a reported sensation of stretching or tension, rather than a reproduction of the chief complaint [15]. Thus, measuring the range of motion and considering symptomatology (i.e., tightness, stretching, pain, paresthesia) may improve SLR sensitivity and reduce false negatives for the diagnosis of SNE-A. This challenges the commonly held belief that SLR is exclusively or commonly a nerve root test [32]. Hamstring tightness is another reported reason for a reduced SLR range of motion. Adding ankle dorsiflexion at the end of the SLR, as was done here, is helpful in structurally differentiating between neural and muscle tissue involvement [33], and assists in ruling out hamstring disorders and ruling in neural involvement.
Palpation is an essential component of the physical exam, yet it remains a controversial skill, as it is highly variable, and it is most reliable when performed at the expert level [34,35]. The practitioners providing treatment in this study had a mean of nine years of experience, combined with hundreds of hours each of hands-on training specific to diagnosis, palpation, and treatment with Manual Adhesion Release®. These are specific skills that require extensive training; therefore, the generalizability of these results may be affected. Palpation for mobility of the sciatic nerve is a procedure that requires a high level of skill, and is of central importance as it aims to directly identify reduced nerve mobility relative to the surrounding deep gluteal structures. This skill has not been adequately tested to confirm its validity and reliability as a non-invasive assessment tool. Nevertheless, the author finds the palpation skill to be adequately utilized by practitioners that have the necessary training and experience.
Despite several limitations, the diagnostic criteria outlined in this study combining assessments of limited SLR range of motion, symptom manifestation, increased symptom intensity upon dorsiflexion, and reduced sciatic nerve mobility assessed by palpation effectively identified a patient population that responded well to Manual Adhesion Release® treatment. Following the diagnosis of SNE-A, applying treatment to the location of fibrous adhesion, and generating tissue tension, was more specific than other conservative care treatments and may be more successful.
These criteria can be applied prior to expensive and time-consuming imaging, injections, therapeutic trials, and endoscopic visualization to potentially improve diagnosis of the presence of adhesions and sciatic nerve entrapment. There is a need for a less expensive, less invasive, and faster point-of-care diagnosis to aid primary and conservative care providers in confirming the important and under-recognized diagnosis of SNE-A. Furthermore, there is a need for well-defined conservative care beyond broad treatment categories, such as neural gliding, soft tissue mobilization, or physical therapy. The Manual Adhesion Release® protocol meets this need, as it is a detailed and specific treatment for SNE-A.
The present study had several limitations. The use of existing data that has been recorded for reasons other than research necessitates dependence on the availability and accuracy of the medical records, and there was no control or comparison group. There is currently no diagnostic gold standard that is both sensitive and non-invasive, so treatment efficacy is heavily relied upon to infer diagnostic accuracy. The patients in this study had other areas treated after reaching maximum improvement with SLR and Manual Adhesion Release® following the SNE-A diagnosis. Therefore, patients were not contacted for long-term follow-up, as additional diagnoses and treatments would have confounded the follow-up results. However, based on these promising outcomes, a prospective controlled study is indicated.
Using the proposed diagnostic criteria, SNE-A was a primary diagnosis in a substantial number of patients with LBP. Based on the reported improvements in the SLR range of motion, reduction of pain, and perceived improvement, Manual Adhesion Release® treatment appears to be a promising conservative treatment for SNE-A. Future prospective research comparing Manual Adhesion Release® to usual treatments and placebo are necessary to further inform clinicians regarding current diagnostic and therapeutic options to improve patient care and to reduce the expense and burden of surgical procedures to treat SNE-A.
I am grateful to Matthew Lytle, Carl Nottoli, Ryan Pribble, Cody Scharf, and Seth Schultz for providing the clinical dataset, and to Jamie Hansen for organization and administration of the data. I would like to thank Editage (www.editage.com) for English language editing.
The full dataset is included within the article.
The author is the president of Integrative Diagnosis LLC, the organization that developed Manual Adhesion Release® and that holds its registered trademark.
There was no funding for the current study.