Displaced intracapsular hip fracture is a common orthopaedic presentation with a high degree of associated morbidity and mortality. These injuries are commonly managed with internal fixation, hemiarthroplasty or total hip arthroplasty. Hemiarthroplasty is a quick procedure with low complication rates, however in active patients is associated with acetabular erosion and pain sometimes necessitating secondary conversion to total hip arthroplasty (THA). At present, experienced operator preference remains the gold standard for determining the suitability for primary THA in these patients. The aim of this study was to re-evaluate the factors associated with failure of hip hemiarthroplasty secondary to acetabular erosion by comparison with Rockwood, et al's., Clinical Frailty Scale (CFS).
Between January 1996 and March 2014 there were 21 patients who had primary hemiarthroplasty and subsequently required revision to total hip arthroplasty due to acetabular erosion. 15 were included in the study for comparison against a control group who only underwent primary hemiarthroplasty. The CFS was retrospectively applied to all patients according to their documented premorbid function. The groups were compared using Student's t-test and the chi-squared test, and sensitivity and specificity for the CFS in predicting failure of hemiarthroplasty due to acetabular erosion was calculated at each CFS score. Further comparison was made with CFS + age.
The mean CFS of patients who underwent revision total hip arthroplasty (3.5 ± 1.4) was significantly lower than those who did not require revision surgery (5.4 ± 1.4; p = 0.001). Accuracy in predicting failure of the hemiarthroplasty was optimised in patients classified CFS- 1-5 with CFS + age ≤ 86 which reported a specificity of 85.7% (95% CI 0.561-0.974) and a sensitivity of 93.3% (95% CI 0.660-0.997) and overall accuracy of 90%.
This study shows that CFS in conjunction with age is a quick tool which may be used to guide clinicians in predicting failure of hemiarthroplasty in the elderly with displaced intracapsular hip fractures. We recommend a prospective randomised controlled study to further evaluate the prognostic role of the CFS in guiding management of acute displaced intracapsular proximal femur fractures.
Hip fracture is a common orthopaedic presentation with a high associated morbidity and mortality [1,2]. The Australian and New Zealand Hip Fracture Registry (ANZHFR) reports hip fractures to be the most costly fall-related injury sustained by older people [2]. After a hip fracture, the current literature reports that only one-third of patients who survive the injury will return their premorbid level of independence [1]. Long-term assistance with routine activities of daily living is required by at least fifty percent of patients, and the remaining group will require residential care [3]. In order to minimise the impact of the insult, careful consideration regarding the definitive treatment of these fractures is essential.
Displaced intracapsular fractures can be managed with internal fixation, hemiarthroplasty or total hip replacement (THR) [4]. Hemiarthroplasty is recognised as a quick and highly standardised procedure offering the advantages of higher stability, lower dislocation rate, pain control, rapid mobilisation and accelerated rehabilitation [5]. However, within patients who present with displaced subcapital proximal femur fracture (PFF), there is a high burden of comorbid osteoarthritis which may necessitate later revision to THR [6]. For the robust elderly patient, THR has demonstrated superior clinical results and fewer complications when compared with hemiarthroplasty in mobile, independent patients after displaced fracture of the femoral neck [7,8]. The decision between hemiarthroplasty and single-staged THR must be weighed against the potential harms of a prolonged and more invasive surgery in addition to the increased risk of immediate and late complications such as dislocation, prosthetic joint infection and the general complications of surgery. There is no clear consensus regarding which operation is most appropriate and this leads to significant variation across institutions [9,10]. The current standard of decision making is subjective and based on the operator's preference.
A study from our centre previously analysed patient factors associated with failure of hip hemiarthroplasty secondary to acetabular erosion. The publication reported that patients who could independently shop, shower and get dressed were more likely to require revision surgery secondary to acetabular erosion and concluded that it may be feasible to objectively identify which patients should receive each type of management. The combination of all three factors reported a sensitivity of and specificity of 80.0% (95% CI 0.513-0.947) and 81.2% (95% CI 0.537-0.950) respectively. The study concluded that the above factors in addition to younger age and higher baseline mobility were all likely factors that increase the probability of failure of the hemiarthroplasty and the need for revision surgery [11].
Since the aforementioned publication, Rockwood, et al's., Clinical Frailty Scale (CFS) has developed traction from gerontological research and entered the surgical sphere as a mode of predicting complications and outcomes from surgery in the elderly population [12]. Recent research has shown yield in predicting mortality after transcatheter aortic valve replacement, coronary artery bypass grafting, burn injuries and colorectal surgery [13-16]. The Clinical Frailty Scale seeks to discriminate between degrees of frailty by observing an individual's fitness, mobility and ability to perform the higher and lower order activities of daily living. The authors of this publication have previously demonstrated the utility of a retrospectively applied clinical frailty score in predicting 30-day and one-year mortality after proximal femur fracture [17]. The Clinical Frailty Scale has been shown to be a valid retrospective tool with retrospective and prospective scores demonstrating high agreement and accuracy in acutely hospitalised patients [18].
The aim of this study is to re-evaluate the factors associated with failure of hip hemiarthroplasty secondary to acetabular erosion by comparison with Rockwood, et al's., Clinical Frailty Scale.
We performed a repeat analysis of cases sampled for a single-centred retrospective case-controlled study in Perth, Western Australia. Between January 1996 and March 2014 there were 1955 patients who had primary hemiarthroplasty with 365 patients requiring revision hemiarthroplasty of hip. The radiographs of these cases were reviewed to confirm 21 cases of revision hemiarthroplasty to total hip replacement due to acetabular erosion. Medical records for these patients were recalled and the revision reason was re-analysed to confirm acetabular wear. Key words implicated in acetabular wear include "erosion" and "pain on weight bearing". Cases revised due to infection, loosening of prosthesis and recurrent dislocations were excluded leaving 15 patients (revision group). The 15 hemiarthroplasty cases of similar age, gender, fracture pattern, ASA grade and premorbid function were selected to compare (control group). These cases were reviewed to confirm that they were not subsequently revised later.
The clinical frailty score was retrospectively applied to cases according to the orthopaedic, orthogeriatric and allied health admission notes which detailed cognitive function, medical comorbidities, admission residence, premorbid fitness, and the degree of services/supports implemented in their place of residence. The SHARE-Clinical Frailty Scale algorithm was utilised to minimise variance and determine the clinical frailty score [19]. The CFS grade was evaluated by two independent reviewers who were blinded to the patient groups. Patients who received different frailty scores from the reviewers were re-evaluated and discussed to establish an agreed frailty score. If consensus was not reached, the lower frailty score was assigned.
All statistical analysis was calculated using IBM SPSS statistics 22 (SPSS, Inc). Continuous variables were expressed as mean ± standard deviation. Nominal variables were expressed as percentage of the total. Nominal demographic data was compared with two-tailed unpaired Student's t-test, and significance was set at p < 0.05. Categorical data was compared using chi-squared regression. Effectiveness in predicting revision was determined by calculation of the sensitivity and specificity at each clinical frailty score threshold and further comparison was made with younger age + CFS.
There were a total of 30 patients included in this study. 15 control group patients underwent hemiarthroplasty only and 15 patients who had a hemiarthroplasty subsequently underwent revision surgery to a THR due to acetabular erosion.
The mean age of the control group was significantly higher than the mean age of the revision group and was reported as 85.7 ± 5.4 years with a range of 77 to 95 years, whereas the mean age of the revision group was 76.4 ± 8.3 with a range of 60 to 91 years (p = 0.001). Both the control and revision group reported a higher proportion of female cases with 9/15 and 11/15 cases being female respectively (p = 0.439).
The Clinical Frailty Scale scores are displayed for each group in Table 1. The control group reported a mean frailty score of 5.4 ± 1.4 and a mode of CFS 6 whereas the revision group reported a significantly lower mean frailty score of 3.5 ± 1.4 (p = 0.001) and a mode of CFS 3. There were no cases with frailty scores of 1 or 2 in the control group and only one case with CFS > 5 in the revision group. The sensitivity and specificity of the CFS in predicting revision surgery at each CFS score threshold is reported in Table 2. The sensitivity and specificity for predicting failure of the hemiarthroplasty due to acetabular erosion were inversely related, and the accuracy of appropriately sorted positives and negatives cases was optimised with a threshold of CFS ≤ 5 (77%).
Table 1: Demographic factors of the control and revision group. View Table 1
Table 2: CFS score of the control and revision group. View Table 2
Further comparison with summation of CFS + age also demonstrated a statistically significant difference in the mean score of 91.1 ± 5.4 for the control group and 79.9 ± 8.3 for the revision group (p < 0.001). The overall mean CFS + age of the sample was 85.5 ± 8.9 and the distribution of this sample is visualised in Figure 1. The utility of CFS + age was optimised at a score of ≤ 86 with a reported sensitivity of 86.7% (95% CI 0.583-0.976), specificity of 80.0% (95% CI 0.513-0.947) and accuracy of 83.3%.
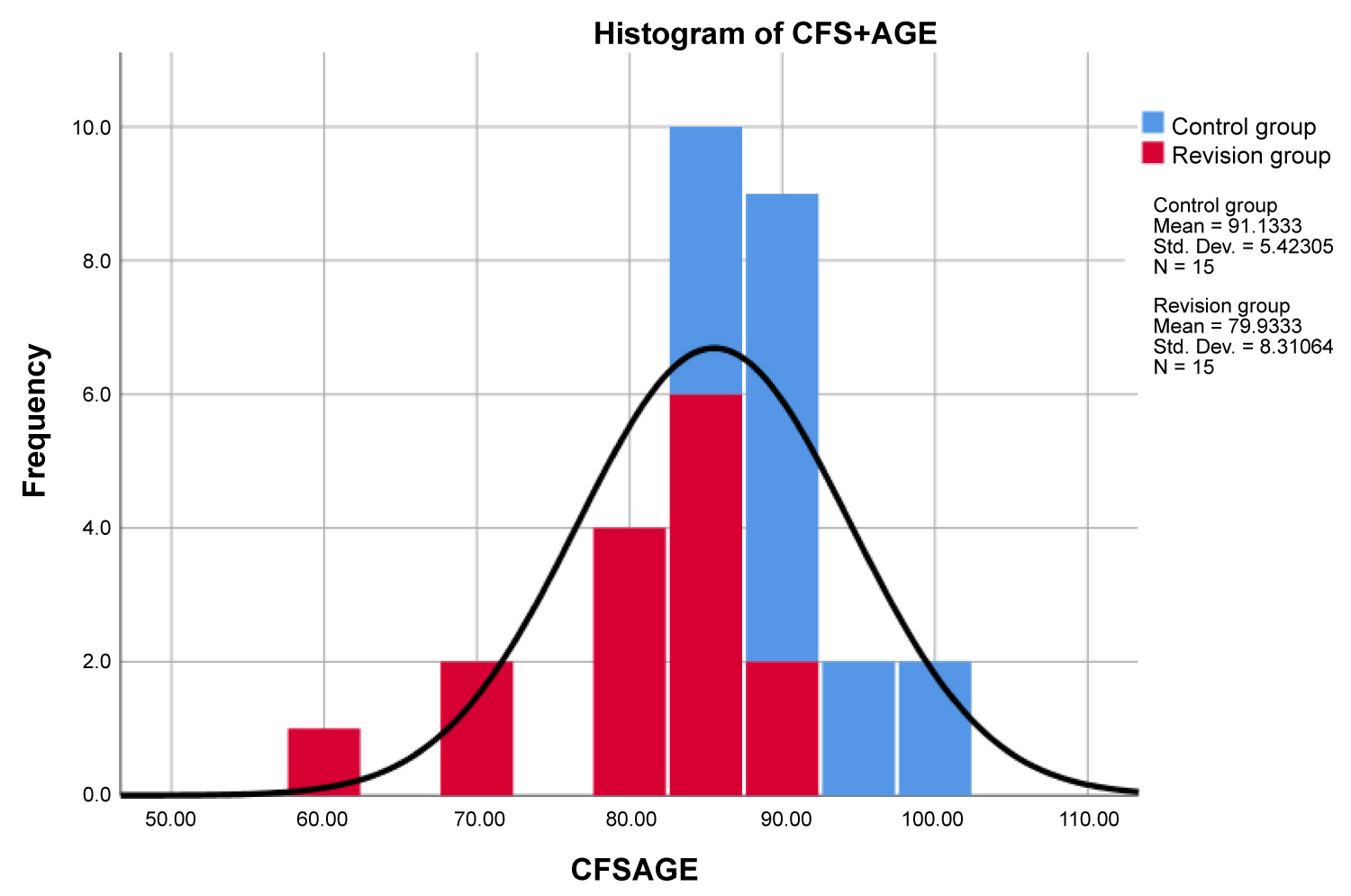 Figure 1: Distribution of CFS + age scores across the control and revision groups.
View Figure 1
Figure 1: Distribution of CFS + age scores across the control and revision groups.
View Figure 1
This retrospective review has demonstrated the potential prognostic value of the Clinical Frailty Scale in predicting failure of hemiarthroplasty due to acetabular erosion after displaced subcapital PFF in the elderly population. Comparison between the control and revision group demonstrated a significantly lower degree of frailty in the revision population (Table 1).
Within the CFS the key factor that discriminates 'non-frail' (CFS 1-3) from 'frail' (CFS 4-9) is the capacity for independent mobility and exercise. It is therefore intuitive that there is a higher proportion of non-frail cases within the revision group as mechanical use of the joint ultimately results in acetabular erosion and hemiarthroplasty failure necessitating revision. There were only two cases within the revision group that were classified as CFS 1 or CFS 2 and this is explained by operator preference resulting in other robust patients being managed with primary THR and therefore not included within this sample of patients who underwent management with primary hemiarthroplasty. Uncoincidentally the mode of the revision group (CFS 3) illustrates the difficulty of discriminating non-frail from frail in the elderly population.
At the institution where this study was undertaken, the standard for deciding the surgical management of subcapital PFF is consultant operator preference. Between the period from January 1996 to March 2014 there were over 1955 patients who underwent hemiarthroplasty. Of these, the database search only yielded 21 cases that required revision surgery (1.07%). This affirms that experienced operator preference is a highly specific method for determining the suitability of THR for displaced subcapital PFF.
During the period of the study the frequency of management with hemiarthroplasty was 10 times greater than management with THR at initial presentation. It follows that a test for determining management would need to be highly specific to avoid performing unnecessary prolonged and invasive surgery, and highly sensitive to accurately predict failure of the hemiarthroplasty. At CFS 1-2 the specificity is 100% (95% CI 0.747-1), however the sensitivity is too low to be clinically useful. At CFS 3 the specificity in this patient group is 86.7% (95% CI 0.584-0.977), and the sensitivity is improved to 60% (95% CI 0.329-0.825).
The sensitivity of the CFS in predicting failure of hemiarthroplasty increased with the frailty score (Table 3). The single case within the revision group classified as CFS 7 was young and independent of mobility but was classified as frail due to severe Alzheimer's dementia. Exclusion of this case would yield 100% sensitivity for predicting failure of the hemiarthroplasty at CFS ≤ 5, however the corresponding specificity is similarly weak at 60%. By comparison of CFS + age at a threshold score of ≤ 86 a sensitivity of 86.7% (95% CI 0.583-0.976) and specificity of 80% (95% CI 0.513-0.947) is yielded with an overall accuracy of 83%. Of note, there were three patients within the control group who had CFS + age ≤ 86 but had CFS scores > 5 and therefore hemiarthroplasty may have still been the appropriate prosthesis for these patients. As such it follows that a patient classified CFS 1-5 and CFS + age ≤ 86 may benefit from primary THR. With this criteria a sensitivity of 85.7% (95% CI 0.561-0.974) and a sensitivity of 93.3% (95% CI 0.660-0.997) is achieved and overall accuracy of patient group assignment is 90%.
Table 3: Sensitivity and specificity of the Clinical Frailty Scale in predicting revision with different score thresholds. View Table 3
The retrospective design of this study underestimates the sensitivity and specificity of the CFS for predicting failure of hemiarthroplasty due to acetabular erosion. If the CFS were to be utilised as a test to predict failure of the hemiarthroplasty, it would be utilised at the time of the surgical decision, and as such the retrospective study is only inclusive of the patients who were not appropriately sorted by operator preference. Given this limitation and the small sample size, the criteria presented here would be most appropriate utilised to identify patients who have had operator preference hemiarthroplasty and should be followed-up regularly to observe for acetabular erosion and arrange proactive elective revision surgery.
This study has demonstrated the potential for CFS to be utilised as a way to guide surgical decision making by objectively identifying patients who may be suitable for a THR. Further studies would benefit from prospectively assigning CFS scores and considering a randomised control trial to compare CFS against operator preference to compare the accuracy of both approaches. The CFS holds considerable advantage in being readily calculable at the bedside and providing reliable patient group assignment over operator preference.