Pain in Marfan syndrome is common, although frequently under diagnosed and undertreated. Few studies have investigated the treatment of pain symptoms in Marfan syndrome and no study has reported on the use of opioid therapy in this patient population. This study aims to characterize the use of pain treatment interventions, including opioid use in individuals with Marfan syndrome. We hypothesize that opioid use would be associated with worse pain and greater psychological burden, and that pain-related disability would moderate opioid use.
Individuals with Marfan syndrome completed an online questionnaire assessing pain severity, psychological burden, and pain management therapies. The sample reporting pain (N = 218) were 74.5% female, average age ranging from 35 to 44 years, predominantly white (91%), and majority are employed (53%).
Participants frequently use a combination of pharmacological and non-pharmacological therapies, and the most commonly used treatments are over the counter medications and opioid medications. Use of opioids, and non-opioid medications, but not non-pharmacological treatments, increases with pain severity. Individuals who use opioids (34%) report more severe and frequent pain compared to non-opioid users, perceive greater pain-related disability and psychological burden, and more frequently utilize the emergency department.
Pharmacological and non-pharmacological treatment for pain in Marfan syndrome is common, although psychotherapeutic interventions are significantly underutilized. Further studies are needed to address pain management in Marfan syndrome. Pain therapies should be multimodal and include adjunctive interventions that reduce pain and suffering, improve function, and enhance quality of life in this patient population.
Narcotics, Opioids, Pain disorder, NSAIDs, Pain management
MFS: Marfan Syndrome
Marfan Syndrome (MFS) is an autosomal dominant connective tissue disorder caused by mutations in the gene encoding Fibrillin-1 (FBN1). Diagnosis is based on the revised Ghent criteria [1]. MFS is a common Mendelian syndrome with a prevalence of 1 in 5000, no predilection for gender or ethnicity, and wide phenotypic variability [2]. The syndrome involves multiple organs affecting cardiovascular, pulmonary, ocular, nervous, and musculoskeletal systems. Pain symptoms are common and numerous in MFS and may arise from various sources. Musculoskeletal involvement is characterized by bony overgrowth, reduced bone mass, reduced muscle mass, and joint laxity [3,4]. Clinical manifestations include, but are not limited to, degenerative arthritis, chest deformities, kyphosis, ligament injuries, and dislocations. While the pathophysiology of pain in MFS is unknown, such clinical manifestations may play a role in its onset and persistence [5].
Pain syndromes in MFS including back and joint painare frequently under diagnosed and undertreated [6,7] as clinical care and research efforts have focused on the life threatening cardiovascular manifestations of MFS. Similar to other pain conditions [7], pain in MFS contributes to significant disability, psychological burden [8-10], and reduced quality of life [8,11]. To our knowledge, only one study has investigated the treatment of pain symptoms in MFS [6] and no studies exist on the use of opioid therapy in this patient population. The efficacy of opioid therapy in chronic pain conditions is sparse [12]. Therefore, it is essential to better characterize the use of pain treatments in this patient population.
Rigorous long-term studies of chronic opioid therapy in neuropathic and musculoskeletal pain are limited [13-18]. Meta-analyses of long-term opioid use reveal marginal clinical efficacy [19]. Many patients report severe pain, reduced function, and distress, and disability while on opioid therapy [20-23]. Both opioid use and chronic pain are associated with higher rates of mood disorders and poly pharmacy [24-28]. Initiation of opioid prescribing is associated with higher baseline levels of pain, psychological distress, and unhealthy lifestyles [29-34]. While opioid use is a risk for long-term disability and poor physical, mental, and cognitive functioning [35-38], evidence also suggests that chronic back pain patients who report higher functional disability scores, greater disability, and poorer functioning are more likely to be prescribed opioids [39,40]; and disability pension predicts persistent opioid use compared with short-term opioid use [41]. Studies show that opioid therapy for pain conditions which are associated with significant psychological burden (i.e., chronic low back pain [42], daily headache [43], and fibromyalgia [44]) is common. As previously stated, no data exist about opioid prescribing practices in individuals with MFS.
Pain symptoms frequently occur at the onset of MFS, and individuals with pain at symptom onset tend to have greater pain severity, pain-related disability, and worse physical and mental health [8]. Given the high incidence of pain in MFS and the medical comorbidities and life-threatening complications associated with the syndrome, it is essential to better understand how pain is managed in this patient population. This study aims to characterize the use of pain treatment interventions, including opioid use in individuals with MFS. In addition, we hypothesize that opioid use would be associated with worse pain and greater psychological burden, and that pain-related disability would predict opioid use.
As previously reported [8], 441 respondents completed an online survey over a 15-month period. Since the survey was anonymous, all data was self-report, and not verified by medical record. Inclusion criteria included reported history of MFS; reported family history of MFS [1]; and reported diagnosis by genetic testing, or physical, ophthalmologic, or cardiac exam. Respondents were excluded if they did not report having MFS, did not report a family history of MFS, nor report how diagnosis was made. 245 respondents met criteria. Of the participants who met criteria, 218 (89%) reported having pain and were included in the following analyses. The sample is 74.5% female, average age ranging from 35 to 44 years, predominantly white (91%) and a slight majority is employed (53%).
Approval for the study was obtained by the Johns Hopkins University Institutional Review Board (IRB). The study was advertised as a survey for all individuals with MFS, regardless of whether they had pain. The survey was maintained on The Marfan Foundation website (http://www.marfan.org), from November 2013 to February 2015.
The web-based survey included six sections of 101 total items. Sections included questions about demographics; diagnostic information; pain symptoms and features, change in pain symptoms over time; current symptoms; and quality of life [8]. The survey also included questions about past and current use of pain therapies.
Participants were asked to self-report their sex, age, race, employment status, and place of residence.
Medical history questions were related to MFS and pain - specifically, how MFS was diagnosed, if there is a family history, and frequency of emergency department utilization.
Self-reported clinical pain severity was assessed as the average of patients' ratings of "current pain", "pain at its worst", least pain", and "general pain" using a 5-point scale from [1] mild to [5] excruciating [8].
An 11-point whole number pain scale to describe pain intensity anchored by descriptors of 0 = no pain and 10 = worst pain imaginable. Respondents chose a number that best reflected pain intensity within the past seven days.
Version 1 of the ODI is a well-validated measure of pain-related disability in individuals with acute or chronic pain [45]. ODI assesses pain and 9 items on activities of daily living using a 6-point Likert scale ranging from least disability to greatest disability. Higher scores indicate greater disability.
The SF-12 is a well-validated 12-item questionnaire of physical and mental health status. The scale yields summary scores for both Physical Health (PCS12) and Mental Health (MCS12) - which are calculated as age-specific mean difference scores and then linearly transformed with respect to the general population (with M = 50 and SD = 10). Higher scores in each category indicate a healthier state [46,47].
The BDI is a well-validated frequently used 21-item questionnaire that assesses the intensity of depressive symptoms over the past week using a 4 point Likert scale. Higher scores indicate more depressive symptoms with a total score ranging from 0-63 [48].
The PCS assesses exaggerated negative cognitive and affective responses to pain using 13 items rated on a 5-point Likert scale (0 - not at all to 4 - all the time). Higher scores indicate greater pain catastrophizing [49].
The ISI is a well-validated, 7-item questionnaire that uses a 5 point Likert scale to assess the nature, severity and impact of insomnia. Higher scores indicate more difficulties with sleep [50].
Respondents were asked to identify pharmacological treatments for pain which they have had in the past, but are no longer receiving. They were also asked to identify non-pharmacological treatments that they have had in the past. A separate question assessed if participants are currently taking or receiving pharmacological and non-pharmacological treatments. The question about past use does not inquire about when treatments were used. Data was categorized into past use or present use. Respondents were asked to select from the following treatments. Over the Counter Treatments (OTCs) include Acetaminophen and Non-Steroidal Anti-Inflammatory Agents (NSAIDs); opioids include codeine, fentanyl patch, hydrocodone, hydromorphone, morphine, propoxyphene, and tramadol; non-opioid prescription-only medications include clonidine, corticosteroids, cox-2 inhibitors, gabapentin, lidocaine patch, and topiramate; and herbal supplements. Participants were also able to write-in other pharmacologic treatments used for pain. The survey did not ask about frequency or duration of use. Respondents were asked about the following non-pharmacologic treatments. Procedures include surgical therapies, nerve blocks, corticosteroid injections, spinal cord stimulation, and chemical and radiofrequency ablation sympathectomy. Physical therapy and occupational therapy were considered separate treatments. Psychological pain interventions included psychotherapy, counseling, relaxation therapy, group therapy, behavioral therapy, behavioral management, and stress management, and biofeedback. Complementary/Alternative Medicine Approaches (CAM) include alternative treatments, acupuncture, chiropractor, and self-hypnosis.
Descriptive statistics are used to summarize demographic characteristics of opioid users. Regarding opioid use, analyses compared participants currently using opioids with those not currently taking opioids. A subgroup analysis of opioid users compared those currently using one opioid with those currently using two or more opioids. Similar to previous work [8], psychological variables are reported as median with Interquartile Range (IQR), and compared between groups using the Mann-Whitney U test. Spearman bivariate correlations were conducted to examine the association between pain treatments and pain characteristics. Finally, Hayes' Process macro [51] was employed to examine the potential moderating effect of disability on the relationship between MFS pain severity and current opioid use. An ordinary least squares or logistic regression-based path analytical framework is employed in this macro to analyze statistical models. Model 1, for simple moderation was used in the current analyses. We controlled for confounding variables, based on differences between opioid and non-opioid use groups, including number of pain sites, number of MFS symptoms, physical functioning, pain catastrophizing, insomnia and depressive symptoms. All respondents who completed psychological questionnaires (N = 142) were included in analysis. Data were analyzed using SPSS (IBM statistics, Version 24).
The 218 participants reported using a range of pharmacological and non-pharmacological pain treatments that are presented in Table 1. The majority reported using Over-The-Counter Medications (OTCs) and opioid medications for pain management. Less commonly used strategies included physical therapy, complementary/alternative medicine approaches, psychological pain interventions, and procedures. The most commonly used past procedures included corticosteroid injections (21.6%), surgeries (21.6%), nerve blocks (8.7%), and spinal cord stimulation (6.4%). Non-opioid medications, other than OTCs, were less commonly reported than opioids and included gabapentin, lidocaine patch, and corticosteroids (Table 2). Supplements were more commonly used than topiramate, cox-2 inhibitors, pregabalin, and antidepressant medications (i.e., tricyclic antidepressants and serotonin-norepinephrine reuptake inhibitors). For all pain therapies, use was more common in the past than the present (Table 2).
Table 1: Current and past use of pain management treatments for pain in MFS. Individuals may report use of more than one treatment. View Table 1
Table 2: Current and past opioid and non-opioid pharmacological therapies for pain in MFS (n = 218). Individuals may report use of more than one treatment. View Table 2
As we previously reported [8], the majority of respondents had pain in more than one location. Respondents who identified pain in more locations reported greater pain severity (r = 0.31, p < 0.001). Use of pain treatments increased with sites of pain (r = 0.40, p < 0.001) and with pain severity (r = 0.26, p < 0.001). Use of opioids and non-opioid medications each increased with increasing pain locations; greater pain severity or pain-related disability. Non-pharmacological treatments increased with pain locations (Table 3).
Table 3: Correlation of pain sites, pain severity, and pain-related disability with number of current pain treatments. View Table 3
Demographic information is reported for those who reported current opioid use (N = 74) and those who did not (N = 144) in Table 4. Older adults (p = 0.02), the unemployed (p = 0.045), individuals with a greater number of Marfan symptoms (14.3 ± 4.8 vs. 11.3 ± 5.4, p < 0.001), and individuals reporting a greater number of pain sites (3.1 ± 11.9 vs. 11.9 ± 1.4, p < 0.001) were more likely to report opioid use. Of individuals taking opioids, oxycodone (46%) and hydrocodone (39%) were the most commonly reported opioids, followed by tramadol (31%). The majority of respondents reported taking only one opioid (66%), but a substantial minority reported taking 2 (16%), 3 (8%), or more than 3 (10%) opioids. Opioids were frequently combined with other treatments, including OTCs (62%), non-opioid medications (49%), procedures (28%), physical therapy (31%), psychological pain interventions (26%), and CAM (21%). Occupational therapy (4%) was the least utilized treatment. Opioid users reported using an average of 3.5 ± 1.8 pain treatments compared with 0.9 ± 1.1 pain treatments for non-opioid users.
Table 4: Demographic information of MFS individuals who are currently using opioids versus those who are not current using opioids. View Table 4
As shown in Table 5, current opioid users reported more frequent pain within the past seven days of survey completion compared with non-opioid users. They also reported greater pain severity (by BPI) and current pain intensity (by NRS). Opioid users were also more likely to report sudden increases in pain, and they were less likely to experience pain remission. Opioid users reported visiting the ED more frequently (3.4 ± 1.6 vs. 2.1 ± 1.5, p < 0.001) in the past 5 years. There were no differences in treatment satisfaction between the groups (p = 0.79). Of note, treatment satisfaction is a complex issue. While no differences emerged between those currently using opioids and not, treatment satisfaction was significantly associated with disability (r = 0.2), pain (r = 0.15), depression (r = 0.3) and catastrophizing (r = 0.2) with worse symptoms correlated with lower treatment satisfaction (Table 4 and Table 5).
Table 5: Comparison of pain frequency, intensity, and quality based on current use of opioids. Reported as either percentage of respondents (%) or mean ± sd. View Table 5
We next examined if opioid users had greater physical and psychological burden than respondents not taking opioids. Comparison of pain-related disability, physical functioning, and psychological factors including mental health functioning, pain catastrophizing, sleep disturbances, and depressive symptoms are reported in Table 6 by current opioid use. Both groups had poor mental health functioning, although there were no significant differences. Opioid users had worse physical health, greater pain-related disability and pain catastrophizing, and worse insomnia symptoms compared with those not currently taking opioids (p's < 0.01). Current opioid users reported that pain affected their sleep including difficulty falling asleep and staying asleep and reported that pain affects feelings of being well rested more so than non-opioid users (p's < 0.01).
Table 6: Comparison of physical and psychological factors for those currently using opioids compared with those who are not currently using opioids. Data presented as median and Interquartile Range [IQR], and N = number of respondents. View Table 6
We conducted a subgroup analysis of current opioid users comparing those who reported using one opioid versus those using more than one opioid. There were no differences between groups for pain-related disability, physical functioning, mental health functioning, pain catastrophizing, sleep disturbances, and depressive symptoms (p's > 0.05) (Table 6).
Pain severity was associated with current opioid use, such that higher pain was associated with greater likelihood of opioid use (N = 142, ß = 1.8, p = 0.02). Greater pain-related disability was also associated with current opioid use (ß = 0.16, p = 0.01). A significant interaction emerged between disability and pain severity in predicting the odds of current opioid use (ß = -0.04, p = 0.04). This interaction is represented graphically in Figure 1, which depicts the simple slope of pain severity for low (-1 standard deviation) and high (+ 1 standard deviation) disability. Simple slopes were tested across levels of disability and only those with low levels of disability revealed an association between pain and opioid use (lower disability: ß = 1.1, p = 0.02). To further examine how the relationship between disability and pain severity may predict current opioid use, we used the Johnson-Neyman technique [51] to evaluate the regions of significance of the conditional effect. This allows for visualization of the range of values within the moderator where the interaction is significant. Figure 2 plots the conditional effect of pain on the probability of current opioid use across values of disability. The region of significance lies where the confidence interval does not include zero. Thus, pain severity is associated with opioid use when pain-related disability is < 29.8. Those with disability scores < 29.8 accounted for over one third (35.2%) of the sample. As shown in Figure 1 and Figure 2, pain severity was significantly related to current opioid use and pain-related disability significantly moderated that relationship, specifically when pain severity and disability were lower (Figure 1 and Figure 2).
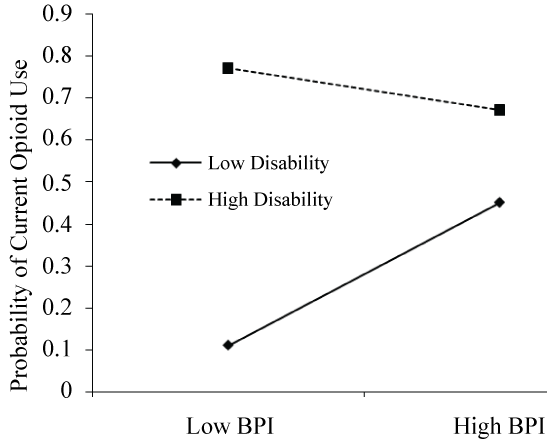 Figure 1: Interaction of disability and pain predicting the probability of current opioid use in 142 patients with MFS. When disability is low, pain drives the opioid decision. However, when disability is high, pain level is not a significant factor in determining current opioid use.
View Figure 1
Figure 1: Interaction of disability and pain predicting the probability of current opioid use in 142 patients with MFS. When disability is low, pain drives the opioid decision. However, when disability is high, pain level is not a significant factor in determining current opioid use.
View Figure 1
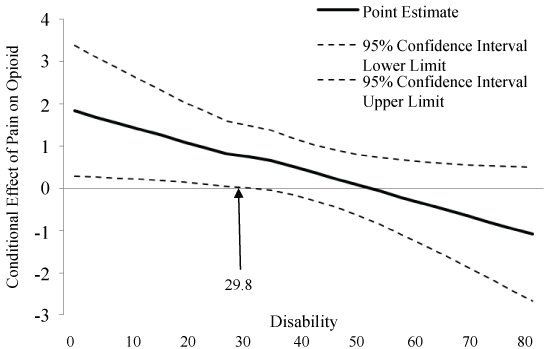 Figure 2: Conditional effect of pain on opioid use across values of disability. The region of significance lies where the confidence interval does not include zero. Thus, pain is associated with opioid use when disability is lower than 29.8. Specifically, when pain severity is low, disability is an important contributing factor to the decision to prescribe or take opioids. View Figure 2
Figure 2: Conditional effect of pain on opioid use across values of disability. The region of significance lies where the confidence interval does not include zero. Thus, pain is associated with opioid use when disability is lower than 29.8. Specifically, when pain severity is low, disability is an important contributing factor to the decision to prescribe or take opioids. View Figure 2
The present study sought to characterize the use of pain therapies in individuals with MFS. We found that MFS individuals are using numerous pain management treatments. Participants frequently use a combination of pharmacological and non-pharmacological therapies, and the most commonly used treatments are OTCs and opioid medications. Uses of opioids and non-opioid pharmacologic treatments increase with pain severity. Consistent with other pain conditions, individuals with MFS who use opioids report more severe and frequent pain compared to non-opioid users and perceive greater pain-related disability and psychological burden.
This paper uniquely contributes to the limited literature of the treatment of pain in MFS [6] as it describes both past and current modalities used for pain therapy, including opioid use and psychological pain interventions. Participants report greater current use of opioid and non-opioid medications compared with physical therapy and other non-pharmacological therapies. Although, past use of physical therapy is the second most commonly used pain therapy. Use of physical therapy for chronic pain is highly variable [52,53] and traditionally utilized as a short but intense course lasting one to two months [54]. Therefore our data agree with current trends in which physical therapy is not a long-lasting pain treatment. Despite our small sample size, utilization of non-pharmacological pain treatments including physical therapy, steroid injection, and spinal cord stimulator were comparable to findings by Nelson, et al. [6]. We also find that psychological pain interventions and occupational therapy are underutilized in this population.
To our knowledge this paper is the first to describe opioid use in MFS. A significant minority of our sample reports concurrent use of more than one opioid and many participants report that they also use OTCs. These findings are consistent with previous findings of opioid use in other chronic pain patients [55]. Similar to other pain conditions, the unemployed [41,56]and older individuals [57,58] were more likely to report opioid use. Contrary to existing literature showing that women are prescribed [25,57,59,60] and use [57] opioids more frequently than men, in our sample of MFS individuals we found no differences in opioid use between sexes. This may be explained by previous findings that, in contrast with numerous pain conditions, no reported sex differences in pain severity exist in MFS [6,8]. We found those with greater pain sites and those with greater number of MFS-related symptoms are more likely to use opioids. This is consistent with findings that chronic pain patients on opioids have more medical comorbidities [61], poorer health [56,62], and more pain conditions [63] compared with non-opioid chronic pain patients.
Extant literature documents that opioid users report severe pain [64], and have higher healthcare utilization compared with non-opioid users [38,65,66]. Our findings suggest that similarly to other chronic pain conditions, MFS individuals who use opioids report more intense, frequent, and non-remitting pain. Not surprisingly, the number of opioid medications increased with pain severity. Pain severity was also associated with increased use of non-opioid pharmacological treatments, but not non-pharmacologic treatments. Our data also suggest that MFS individuals who use opioids visit the emergency department for pain-related problems more frequently than non-opioid users. Further research is needed to determine if the MFS population, similarly to other pain conditions, uses the emergency room for pain management [33].
Generally chronic pain patients are more likely to be treated with opioids if they have greater self-reported disability, poorer function, and greater distress [39,40,42]. Opioid initiation for chronic pain is also associated with comorbid psychiatric conditions [28,67]. Our sample of MFS individuals using opioids reported greater insomnia symptoms and pain catastrophizing compared with non-opioid users. Concordant with extant literature on chronic opioid use [56,68], they also reported greater pain-related disability and worse physical functioning. In our sample of MFS individuals, pain-related disability significantly moderated the pain severity-opioid use relationship. Specifically, when pain severity is low, disability is an important contributing factor to the decision to prescribe or take opioids. While the association between opioid use and disability in this MFS study is based on a small sample size, our data are consistent with previous data showing a strong relationship between opioid use and disability for individuals with pain [39,40,42]. Longitudinal studies are needed to further characterize the relationship between pain and disability in this population. A strength of our study was the moderate sample size and group comparisons using well-validated and commonly utilized assessment measures. The overall incidences of pain treatments were consistent with other pain conditions supporting the generalizability of our study to pain in MFS. One limitation of our methodology is the use of an anonymous online survey which may lead to selection biases as previously described [8]. We did not confirm pain therapies with a physician or medical records. Many limitations stem from our treatment assessments. We did not assess duration or frequency of treatments, collect doses of medications, or differentiate route of use (i.e., oral, intravenous). We did not assess acute (i.e., peri-operative pain management) versus chronic use of pharmacotherapy. While we included a thorough list of pain therapies, we did not assess drug classes including, but not limited to, pregabalin, serotonin norepinephrine reuptake inhibitors, tricyclic antidepressants, or anti-epileptic drugs. Our data likely under represents use of these medications for pain treatment. It is also important to note that use of opioid therapy may not reflect actual prescription of opioids by a provider, but may in fact be more reflective of individual usage patterns. Finally, as this is a cross-sectional analysis we were unable to determine the temporal relationship of pain, pain-related disability, psychological factors, and pain therapies and we were unable to classify opioid use as acute or chronic. Given substantial evidence that pain is a significant problem in MFS, longitudinal studies are needed to evaluate the efficacy of pain therapies in MFS and further studies are indicated to determine reproducibility of data using data from medical records.
Pharmacological and non-pharmacological treatment for pain in MFS is common. After over the counter medications, opioids are the second most frequently utilized pain therapy. While no studies have adequately evaluated the risk of opioids in individuals with pre-existing cardiopulmonary conditions, MFS patients may be particularly susceptible to side effects of opioids including respiratory depression, constipation, fatigue, and falls [14]. Psychological pain interventions are currently underutilized in this population. As with other chronic pain conditions, pain management should be multimodal and include adjunctive interventions that reduce pain and suffering, improve function, and enhance quality of life in this patient population.
This research was supported by grant NIH K23 NS070933 (CMC), and grant NIH T32 NS070201 (TJS).
Approval for the study was obtained by the Johns Hopkins University IRB.
PDS receives honoraria from Depuy Synthes Spine and Globus, revenues from Lippincott Williams and Wilkins as author, and J Bone Joint Surgery as Deputy Editor. He is Deputy Editor of Spine Deformity. For the remaining authors none were declared.
TJS was involved with statistical analysis, data interpretation, and manuscript preparation. MH performed data collection and analysis. PDS and KAW were involved with design of the study and performed critical revision of the manuscript. CMC was involved with design of the study, data interpretation, and performed critical revision of the manuscript. All authors read and approved the final manuscript.