To examine the association of Bone Mineral Density (BMD) in the prediction of frailty, pre-frailty, osteoporosis, falls and health conditions in elderly men and women.
A four-year prospective study (2009-2013) with 106 elderly, both sexes, aged ≥ 60 years, of the city of Goiânia, Brazil. BMD was estimated using dual-energy X ray absorptiometry. Frailty was assessed subjectively, including the following components: unintentional weight loss, fatigue, and low physical activity, reduction of strength and gait speed.
Mean age was 70 years of age and Body Mass Index (BMI) was 26.7 kg/m2. The mean BMD for women was 1.042 (± 0.11) g/cm2 while for men, mean BMD was 1.169 (± 0.12) g/cm2, (p = 0.000). After adjustments for age and BMI in women, lower BMD values were significantly associated with osteoporosis (p = 0.003), frailty (p = 0.033), and pre-frailty (p = 0.037).
BMD was predictive of frailty, pre-frailty and osteoporosis in women. In men, no associations were established.
Bone mineral density, Frailty, Osteoporosis, Muscle strength, Elderly
Bone health and its relationships with several health outcomes of adults and elderly have been in the spotlight of recent research [1-3], as its consequences affect morbimortality. In literature, there is evidence that decreases in Bone Mineral Density (BMD) are due to the natural ageing process for both sexes, however more prevalent for women [3-6], with consequences such as increased risk of fractures [3,6], falls and incapacities in the female group [2].
Within the last years, the frailty syndrome has been increasingly highlighted due to its complex nature [7], with high prevalence and mortality [8], and its relationship with different health conditions has been investigated [9,10]. The majority of studies has analyzed frailty as a predictor of low BMD and indicate a causal relationship with low BMD [2,3,11]. However, analysis of BMD as a predictor of frailty has not been extensively investigated [12,13]; existing research has presented controversial results. While research finds no association between frailty and BMD in older women, another study observed associations between low BMD and frailty markers such as: low strength and low gait speed in men [12,13].
A plausible explanation for the relationship between frailty and low bone mineral density is that the parameters used as frailty markers in the elderly such as advanced age, weight loss, low body weight, sarcopenia, the low level of physical exercise, and impaired mobility, since mechanical crucial is loading to bone mass maintenance [14]. The research presented herein considers the current context of population ageing, as well as the importance of BMD in the general and bone health of elderly. The results presented herein can add relevant information on BMD and frailty of elderly, according to sex, mainly on those living in countries with great social inequality, such as Brazil. Therefore, the endpoint primary of this study was to verify the association between low BMD and frailty and pre-frailty in elderly, according to sex. The secondary endpoints were the prediction of falls, morbidities, osteoporosis, hospitalizations with low BMD according to sex.
A cohort study is presented herein, which integrates the Elderly Project/Goiânia [15-17] that analyzed several aspects of health conditions of the elderly in community-dwelling settings. The initial sample was constituted of 418 elderly, selected by probabilistic sampling carried out in multiple stages, proportional to the health districts of Goiânia (Midwest Brazil). Data were collected by previously trained interviewers and anthropometrics, at the home of the elderly, with standardized anthropometric measurement techniques. The project was approved by the Research and Ethics Committee of the Federal University of Goiás (Protocol n° 031/2007) and all the seniors signed a free informed consent for participation in the study.
The methodological details on sample calculation and sampling are described in previous publications [15,16].
BMD evaluation involved the random selection of 132 elderly from the initial sample and data were collected in June, 2009. The Dual-energy X ray Absorptiometry (DXA) was performed with the device Lunar DPX-MD PLUS, and the quantification of these values was conducted in the software version 7.52.002 DPX-L. DXA was carried out for the entire body, with the following eligibility criteria: not in use of diuretics, body weight under 110 kg and height under 1.90 meters. Reports were issued by a doctor specialized in clinical densitometry. The DXA equipment was regularly calibrated and all requirements for the exam were fulfilled. Data collection was accomplished in a specialized clinic by a previously trained team. The elderly were contacted by phone to schedule data collection and receive information on the DXA exam procedures: fasting, no use of diuretics, and no rigorous physical activity in the 24 hours previous to the exam.
Investigation of the influence of BMD on different health outcomes required that, in 2013, all elderly (132) that underwent DXA in 2009 were contacted for data collection and follow-up. Up to three phone calls were made per phone number, and in the case of inexistent phone number or change of number, home visits were made. Loss to follow-up was reported when the elderly could not be located by phone or home visit. Follow-up included finally 106 elderly, to whom standardized and validated questionnaires were applied regarding frailty [18].
The following components were evaluated in the frailty questionnaire: unintentional weight loss, fatigue, low physical activity, reduction in strength and in gait speed. According to these components, the elderly were classified in not frail (no component identified), pre-frail (presence of one or two components), and frail (presence of three or more components) [18]. The choice for the subjective evaluation of the fragility was due to the specialized equipment and specific training required for the objective assessment, generating greater difficulty of implementation in primary health care. The subjective evaluation is constituted by a validated instrument for this population and is easy to apply [18].
The outcome variables were: falls, hospitalization, presence and number of morbidities, osteoporosis, frailty and pre-frailty. Falls was evaluated from the following questions: "Have you suffered any fall within the last six months?" Besides these questions, the elderly were questioned about any hospitalizations within the last year ("Have you been hospitalized within the last year?") and presence of diseases including osteoporosis, according to the epidemiological research method utilized previously [19].
The database was structured in EPIINFO® version 7.0, with double entry to check for inconsistencies and validated. The database was transferred to the statistical package STATA/SE 12.0. All analyses were stratified by sex. Student's T test or ANOVA were used to analyze the difference between the mean BMD in the categories of the studied variables, considering a 5% significance level. Multiple linear regressions were carried out between BMD and the outcome variables, adjusting by age and Body Mass Index (BMI). Only those variables with bivariate analysis p-values lower than 0.20 were included in the multivariate analysis models. R2 was calculated for each analysis model to verify variability.
Of the 132 elderly that underwent BMD evaluation in 2009, only 106 were located for follow-up in 2013. Loss to follow-up included 13 deaths, one refusal and 12 elderly that were not located (Figure 1).
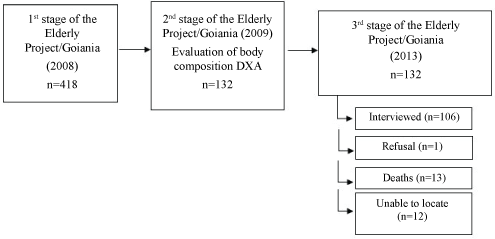 Figure 1: Sample follow-up of the Elderly Project/Goiania.
View Figure 1
Figure 1: Sample follow-up of the Elderly Project/Goiania.
View Figure 1
In the elderly studied in the 2013 follow-up, the mean age was 70 ± 6.40, with mean BMI 26.7 (p = 0.078). Medical diagnosis of two or more diseases occurred for 87.8% of women and 80% of men. Hospitalizations were reported by 45% of women and 44.62% of men. Osteoporosis diagnosis was verified for 50% of women. The prevalence of frailty was 61% for women and 52% for men. The loss of muscle strength was the most frequent frailty criterion in both sexes, with 67.5% in men and 75.3% in women (Table 1).
Table 1: Frequency of falls, frailty criterion and health conditions in the elderly. View Table 1
Mean BMD for women was 1.042 ± 0.11 g/cm2 and for men, 1.169 ± 0.12 g/cm2, with a statistically significant difference (p = 0.000). BMD for men was not statistically associated with falls, number of diseases or hospitalizations. There was, however, an association between BMD and osteoporosis in women, with significantly lower BMD in those presenting osteoporosis (p = 0.003) (Table 2).
Table 2: Association between BMD and falls, and health conditions in the elderly. View Table 2
For frailty and its criteria, no significant differences were observed for BMD in men. However, in women, lower BMD values were associated with reduction in strength (p = 0.023) (Table 3).
Table 3: Association between bone mineral density and frailty and frailty criteria in the elderly, by sex. View Table 3
After multivariate analysis, no association was established between BMD and outcomes for the male elderly, even after adjustments by age and BMI. For women, there was significant association between BMD and osteoporosis (p = 0.003) adjusted by age. After age and BMI adjustments, there was association of BMD with frailty (p = 0.033) and pre-frailty (p = 0.037). Considering the R2 value, the frailty variable was able to explain 43% of BMD data variability in elderly women (Table 4).
Table 4: Multiple linear regression for BMD and evaluated outcomes for women. View Table 4
The results of this study demonstrated that low BMD was a predictor of frailty, pre-frailty and osteoporosis in elderly women. However, BMD was not a predictor of any of the analyzed outcomes for men over 60 years of age. Despite increasing interest of scientific literature in bone health [1-3], the study presented herein contributes with information on the association of BMD with adverse health events, mainly frailty, in female elderly; no association was verified for the male elderly.
Stratified analysis by sex, for the analysis of factors associated with BMD, is important because the process of bone remodeling is affected by alterations in the production of estrogen (which occurs after menopause). Deficiency in estrogen levels results in lower bone metabolic activity, increasing bone reabsorption and consequently progressive loss of trabecular bone [10,20].
Existing research show that low BMD, osteoporosis and fractures are predominant in the female sex [3,5,6,21], however no studies were found on the evaluation of BMD as a predictor of adverse health events in elderly of both sexes [1]. The reduction of sex hormones with advancing age is one of the factors that influence one reduced BMD in both men and women [22]. In females the estrogen decline, resulting from menopause, is the main reason for this loss of BMD, as this hormone is responsible for fixing calcium in the bones and maintenance of BMD [22].
The bone loss that occurs in both sexes is quantified through BMD, and utilized to diagnose osteoporosis and also analyze the risk of fractures [22,23]. In this sense, the association observed between low BMD and osteoporosis in women is an expected result. We carry out preliminary analysis of the occurrence of self-reported by elderly fracture and low BMD, but not found association. However, such association is not the objective of the current study.
Still within this context, considering the hypothesis that low BMD can lead to fractures and these lead to hospitalization, one of the analyses carried out was the verification of the predicting capacity of BMD in hospitalizations. Nevertheless, no associations were verified between these variables and no literature data was found on the association of BMD and elderly hospitalization for comparison purposes herein. However, the studies on BMD and hospitalization verify the effect of hospitalization on the reduction of BMD [24,25] and not the effect of BMD on the occurrence of hospitalizations.
One of the highlights of this study was the association between low BMD and pre-frailty and frailty in female elderly. A study with female elderly of a more advanced age group (over 75-years-old) in Sweden did not establish any association between BMD and frailty [12]. Other studies analyzed frailty as a predictor of low BMD [1,3,26] or other outcomes related to bone health, such as falls, fractures and incapacity [2,21]. Research that evaluate relationships between low BMD and frailty, as an outcome or predictor, present associations and divergences. The controversies can be attributed to the complexity in the development of frailty, a multifactorial syndrome related to neuromuscular and neuroendocrine alterations [7,27] that, in turn, cause alterations in body composition. Low BMD is not explicitly presented as one of the triggering factors of the syndrome, as proposed by Fried, et al. 2001 [7]. However, there is evidence in literature that frailty and osteoporosis (consequence of low BMD) share risk factors (age group, sarcopenia, sedentarism, low body weight, consumption of tobacco) and physiopathological mechanisms [27,28]. In the latter, alterations in estrogen and growth hormone levels would be related to osteoporosis, as these are involved in the bone remodeling process, and related to frailty because they contribute with loss of muscle mass and strength [27,29,30]. This information presents biological plausibility as in this study, loss of strength in women was the frailty component associated with low BMD. These divergences can also be attributed to the use of different instruments to identify frailty in these studies (criteria of the Cardiovascular Health Study [7,31]; and subjective models) and to the different locations used to measure low BMD. A study in Korea found that men with sarcopenia were 3.89 times more likely to have low BMD, while women were 1.87 times more likely to have low BMD, which reinforces that loss of muscle strength (from the sarcopenia evaluation criteria) is associated with BMD [28]. However, the present study did not aim to evaluate the influence of BMD on sarcopenia, since such measurements (of bone mass and appendicular muscle mass) were performed simultaneously. Additionally, the evaluation of sarcopenia also takes into account the assessment of muscular function and strength. Since BMD is a variable not included in the cycle proposed by Fried [7] as a variable for predicting the fragility, but with plausibility to influence its occurrence, this work had as interest to investigate if low values of BMD were able to influence the future occurrence of fragility.
Another question that must be considered regarding the association between BMD and frailty is the BMI adjustment, as it can act as a modifier in the association [10,32] and therefore BMI must be always considered in multivariate analyses.
Regarding the other studied variables, no association was observed between BMD and other comorbidities. The elderly presented high proportions of chronic diseases, which are usually diagnosed before the age of 60. There is other evidence that the presence of chronic diseases such as diabetes, hypertension and dyslipidemia [9], obesity and metabolic syndrome [10] were not associated with low BMD in post-menopause women, but the study presented herein is the first to carry out such evaluation for elderly men.
Loss to follow-up of approximately 10% can be mentioned as a possible limitation of this research, due to deaths within the period of four years. These deaths could be related to adverse health events. However, it was not possible to verify the causes of death due to ethical issues and family embarrassment. Other limitation this research is that the sample size, but due to depreciation of bone mineral density in women is more evident than in the opposite sex, the sample of the current study was able to predict the association among women.
It is important to consider the applicability of the research in the context of health service practices, and therefore this research chose subjective frailty measurements due to its easiness of application, low cost, reproducibility, and accuracy [18,33,34]. These qualities are important both in the context of the reality of many health units as the entry door is primary attention to health, where ambulatory patients do not undergo complex exams, and for the development of epidemiological studies. The objective evaluation of frailty demands specific equipment and trained personnel, which are not always available and accessible at health services [1,34], especially considering the reality of less developed areas of Latin America, Africa and Asia.
After a four-year follow-up, it was concluded that BMD was a predictor of osteoporosis, frailty and pre-frailty in elderly women. It is suggested that other studies are carried out on these aspects, as throughout time, bone health (BMD) can act on other important adverse outcomes in the health of the elderly. Research in this area contributes with valuable information, both for prevention actions and clinical treatment. Therefore, in the current scenario of population ageing, analysis of bone mass and frailty are important in the areas of prevention and public policies for the health of the elderly.
EAS participated in planning the study design, designed the study, prepared and analysed the manuscript, carried out the statistical analysis and participated in the writing of the manuscript. ECSSA collected the data, carried out the statistical analysis and participated in the writing of the manuscript. VP designed the study, collected the data and participated in the writing of the manuscript. All authors participated in interpreting the statistical analysis, read, added comments and approved the final manuscript.