Turner's syndrome is characterized by the partial or complete absence of one of the sex chromosomes in women in association with one or more clinical exposures. Molecular techniques such as polymerase chain reaction (PCR) are useful when it comes to complementing the genetic approach, as they present greater sensitivity in recognizing mosaicism. Considering the high percentage of mosaicism and the possible clinical impact of the presence of Y chromosome in these patients, we proposed in this study to develop multiplex PCR (mPCR) to assess the presence of Y. DNA samples were collected from 109 patients. Primers for mPCR were built with the help of the MPprimer program to evaluate possible interactions between them. Two primers present on the Y chromosome and one on the X chromosome. The amplified samples were evaluated by conventional electrophoresis on 1.8% agarose gel and subsequently by capillary electrophoresis. 12.8% of patients were found with positive molecular markers on the Y chromosome. This study allowed the development of a mPCR capable of detecting fragments of the Y chromosome in these patients. In addition to the practicality of its use in diagnosis, this technique can be used to detect these same fragments in other tissues.
Turner Syndrome (TS) is an entity clinically characterized by the combination of sexual infantilism, short and winged neck, cubitus valgus, short stature and gonadal dysgenesis, whose frequency is approximately 1:2,500 female newborns [1,2]. In approximately 40 to 60% of patients with TS, the karyotype is 45, X and the others have mosaicism [3]. The presence of Y chromosome material in individuals with gonadal dysgenesis is associated with an increased risk (15-20%) of developing gonadal tumors [4].
The karyotype is a method performed routinely for the diagnosis of TS and to evaluate the presence of the Y chromosome. However, this method may fail in cases where the Y chromosome or fragment is present only in a small proportion of cells or even in marker chromosomes containing specific regions of Y [5]. Molecular techniques such as PCR (polymerase chain reaction) are useful to complement the genetic approach in TS, as they have greater sensitivity in recognizing mosaicism, detecting sequences derived from the Y chromosome between 0 - 61% [6-8]. This variability may be due to the molecular methods used, patient selection criteria, different tissues examined and the selection of specific primers on the Y chromosome [9].
Taking into account the high percentage of mosaicism and the possible clinical impact of the presence of fragments of Y chromosome in TS, we proposed, in this study, to develop multiplex PCR (mPCR) to assess the presence of Y chromosome in patients with TS and X chromosome monosomy or with presence of a marker or ring chromosome towards the conventional karyotype.
A total of 142 patients diagnosed with TS were initially recruited, aged 1-60 years (mean = 22 years, standard deviation 11.7), with a karyotype distribution between 45, X (71.1%, n = 101), 45, X/46, X, r (?) (3.5%, n = 5), 46, X, r (?) (N = 1; 0.7%), 45, X/46, X + sea (1.4%, n = 2), others (23.3%, n = 33), coming from the Pediatric Endocrinology and Clinical Endocrinology Clinic of the Federal University of São Paulo - UNIFESP - EPM. Such study was approved by the Medical Ethics and Research Committee via Plataforma Brasil (CAE: 48099115.0.0000.5505) and a term of clarification and free consent, and consent, was obtained from the parents or guardian of the patient.
DNA samples were collected from 109 patients, with karyotype with X monosomy (n = 101; 92.7%) or 45, X/46, X, r (?) (N = 5; 4.6%), 45, X/46, X + sea (n = 2; 1.8%) and 46, X, r (?) (N = 1; 0.9%).
Blood samples (3 ml) from patients were collected in EDTA tubes and kept at 4 ±C until processing. Genomic DNA was obtained using the modified Bowtell method [10]. The quality and quantification of the total DNA was performed by measuring the absorbance at 260 nm in a spectrophotometer (Nanodrop 2000). Two fertile and healthy 46, XX and 46, XY individuals were invited as laboratory control for the absence and presence of Y chromosome markers, respectively. For the calculation of the sample size, the prevalence of fragments of the Y chromosome was assumed in 10% [11] of the patients with TS, a confidence interval of 95% and imprecision of 6%.
Primers for mPCR were built with the aid of the MPprimer program to assess potential interactions between them [12]. Two primers were designed on the Y chromosome, for the SRY gene (GenBank: X53772.1) and for TSPY (GenBank: X74029.1) and one present on the X chromosome, for the amelogenin gene - AMELX (GenBank: NG_012040.1), as an internal control of the reaction (Table 1).
Table 1: Primers for PCR built with the aid of the MP primer program and using the gene sequences available on GenBank (www.nbci.com). View Table 1
The PCR reaction was performed with a final volume of 25 μl containing 50 to 100 ηg of genomic DNA; 1.5 mM MgCl2, 0.02 mM each dNTP, 0.05 mM each primer and 2.5 U of Taq platinum DNA polymerase (Invitrogen, Brazil), 10X PCR buffer (Invitrogen, Brazil). The conditions for thermocycling were as follows: Pre-denaturation at 95 ±C for 5 minutes, followed by 35 cycles: denaturation at 95 ±C for 45 seconds, specific annealing temperature for each primer pair for 30 seconds, an extension at 72 ±C for 30 seconds, and a final extension step at 72 ±C for 10 minutes.
For amplification, the Veriti 96 Well Thermal Cycler thermocycler (AB Applied Biosystems) was used. PCR was performed separately for each gene, using the annealing temperature determined by the MPprimer software. To select the best annealing temperature for the mPCR reaction, temperatures between 56 ±C to 62 ±C were used. All amplifications were accompanied by a male (46, XY), a female (46, XX) and a negative control (white - Milli-Q water - ultra pure).
The amplified samples were evaluated by conventional electrophoresis on 1.8% agarose gel prepared with TAE 1X, stained with gelRed, along with a DNA fragment size standard of 100 bp DNA Ladder (Invitrogen, Brazil) to an electric field uniform with electric current of 100 volts and 400 mA, for 30 minutes and observed in the transilluminator GEL DOC EZ Imager (BIO RAD), being subsequently performed the photographic recording of the “bands” obtained. The amplicons obtained were paired with the allelic references for each amplified locus aimed at confirming that the PCR products had the expected sizes.
Primers for SRY, TSPY and AMELX were marked fluorochromes for use in capillary electrophoresis. Data were collected automatically in the Data Collection program (Applied Biosystems) and analyzed manually with the support of the GeneMapper 4.0 program (Applied Biosystems).
For the standardization of mPCR, variables such as type of DNA polymerase, magnesium concentration, primers, in addition to ringing temperature were analyzed, since mPCR is much more sensitive to these variables than PCR performed with only one pair of primers. The final mPCR reaction was standardized with a volume of 25 μl containing 100 ηg of genomic DNA; 1.5 mM MgCl2, 0.02 mM of each dNTP, 0.05 mM of the primer for the TSPY gene and double for the other primers and 2.5 U of Platinum® Taq DNA Polymerase, 10X PCR buffer and ultrapure water. The reaction was carried out with the following parameters: 5 minutes of denaturation at 95 ±C, followed by 35 cycles of 45 seconds at 95 ±C (denaturation), 30 seconds at 58 ±C (annealing), 30 seconds at 72 ±C (extension) and ending with 1 final extension cycle for 10 minutes at 72 ±C. All three primers were amplified specifically in the male control and only the internal control (AMELX) was amplified in the female control, without the presentation of nonspecific bands. For amplification, the Veriti 96 Well Thermal Cycler thermocycler (AB Applied Biosystems) was used. The amplified samples were evaluated by conventional 1.8% agarose gel electrophoresis and, subsequently, by capillary electrophoresis (Figures 1 and Figure 2).
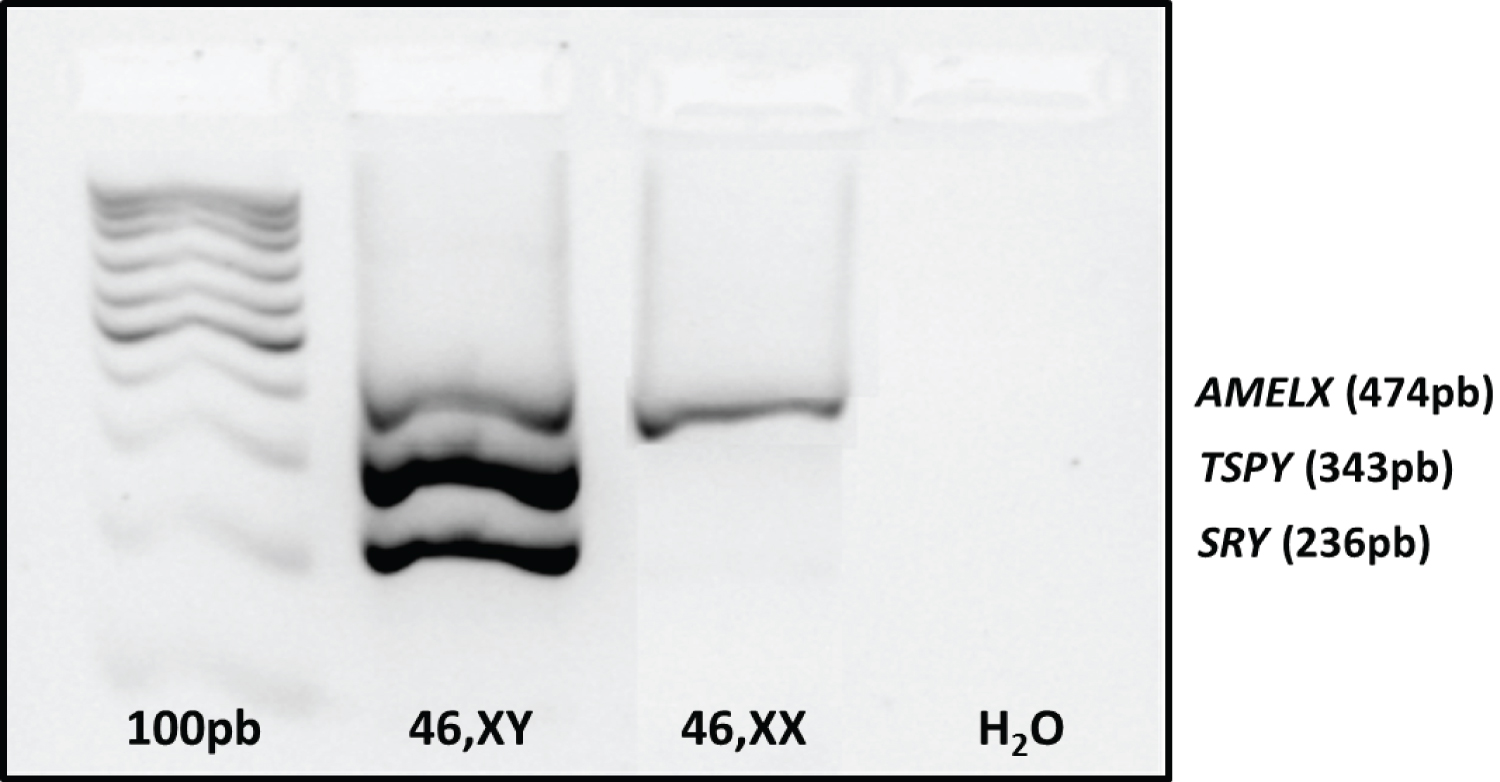 Figure 1: Result of mPCR standardization visualized on 1.8% agarose gel.
Figure 1: Result of mPCR standardization visualized on 1.8% agarose gel.
Genes SRY (236bp) and TSPY (343bp) present on the Y chromosome and AMELX gene (474bp) present on the X chromosome (internal reaction control). Channels 1: ladder 100bp, 2: control 46, XY; 3: control 46, XX and 4: white.
View Figure 1
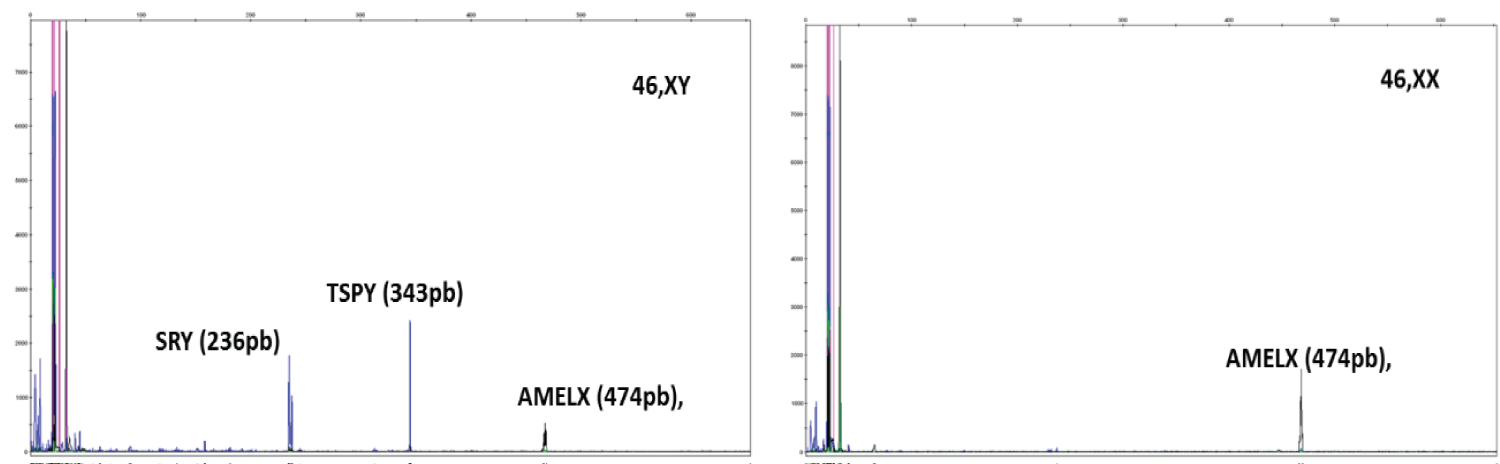 Figure 2: Capillary electrophoresis.
Figure 2: Capillary electrophoresis.
mPCR standardization. Genes SRY (236bp) and TSPY (343bp) present in the Y chromosome (in blue) and AMELX gene (474bp), in black, present in the X chromosome (internal control of the reaction). A) Control 46, XY. B) Control 46, XX. Analysis in GeneMapper v4.0.
View Figure 2
Out of the 109 with TS, 14 (12.8%) patients were found to be positive with the two molecular markers for the Y chromosome.
The main difficulty found for the standardization of mPCR was in adjusting the amplifications, which had bands with low intensity for the SRY and AMELX genes in the agarose gel. The increase in magnesium concentrations of these primers and subsequent change in the type of DNA polymerase from conventional to hotstart, allowed solving this problem. We would have liked to have developed an mPCR with a larger number of fragments of the Y chromosome, but it was not possible due to the great competition between the primers.
The mPCR standardized in this work was able to detect the presence of fragments of the Y chromosome in 12.8% of patients with TS, similar data found in other studies [7,9,13]. Of the 109 patients evaluated with TS, 9.9% (n = 10), presented the 45, X karyotype. Thus, it was shown that the karyotype, despite being considered the gold standard for the laboratory diagnosis of TS, has a low sensitivity (17.6%), to identify Y chromosome mosaicism, in relation to mPCR.
mPCR has a great impact on the economy, with regard to the reduction of reagents and DNA samples used in the reactions, in the time used to carry out the reactions, in addition to the considerable increase in the information obtained per unit of time [14].
Allied to the mPCR, the analysis of the results by capillary electrophoresis, allowed the fragments to be detected and analyzed simultaneously in, resulting in greater precision in the allelic detection, using the same tube as the test sample, with cost reduction and analysis time, in addition to minimization errors inherent to manual and visual analysis on horizontal electrophoresis gel [15].
In conclusion, this study allowed the development of an mPCR capable of detecting fragments of the Y chromosome in patients with TS. In addition to the practicality of its use in diagnosis, this technique can be used to detect these same fragments in other tissues.
The authors declare no conflict of interest.