Vitamin K2 and phosphatidylcholine are two common drugs in clinical treatment. Studies carried out in the past several years demonstrated vitamin K2 and phosphatidylcholine could separately inhibit hepatocarcinogenesis. In this study, we sought to investigate the synergy of vitamin K2 and phosphatidylcholine and the potential mechanism.
Multiple assays were performed to evaluate the effect of combination administration in vitro and in vivo. Then microRNA microarray, bioinformatics analysis and western blot were performed to explore the potential mechanism of drug action.
In vitro, combined administration of vitamin K2 and phosphatidylcholine for 72 hours showed significant anti-tumor effect in four HCC cell lines (Hep-3B, Hep-G2, Huh-7 and SMMC-7721). in vivo, tumor growth was significantly suppressed in the treated group. According to microRNA microarray and bioinformatics analysis, miR-16 was significantly up-regulated and WNT signaling pathway was strongly correlated with the process of anti-tumor. Then western blot analysis indicated that low-expression of WNT3A, p-β-catenin and Bcl-2 accorded with the assumption of miR-16's function.
At last we inferred, given together, vitamin K2 and phosphatidylcholine exhibited synergy against hepatocarcinogenesis via miR-16 regulating. However, further study is needed to confirm these regulatory relationships.
Hepatocellular carcinoma, MiR-16, Bcl-2, WNT3A
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide and the fifth leading cause of cancer-related death [1]. Proliferation and metastasis of cancer cells remains the main cause of HCC-related death. Better knowledge of changes in gene expression during proliferation and metastasis may lead to improvements in the treatment of HCC. MicroRNAs (miRNAs) are a class of small (approximately 18-24 nt) nucleic acids that negatively regulate gene expression. This novel class of molecules modulates a wide array of growth and differentiation processes in human cancers [2,3]. In HCC, consistently deregulated miRNAs were identified, and they played important roles in diagnosis and treatment [4-6].
Vitamin K2 and phosphatidylcholine (PC) are two common drugs in clinical treatment. Studies carried out in the past several years demonstrated that vitamin K2 could inhibit growth of human hepatic cancer [7-11] and a choline deficient diet could itself cause hepatoma in rats [12-14]. In our study, we examined whether combined administration of vitamin K2 and PC could inhibit hepatocarcinogenesis in vitro and in vivo. To explore the mechanism of the anti-tumor effect, miRNA microarray and bioinformatics analysis were performed to search for the potential correlated miRNA and signaling pathway. Lastly key proteins in the process were detected by western blot to identify the inference.
HCC cell lines (Hep-3B, Hep-G2, Huh-7 and SMMC-7721) were purchased from Shanghai Cell Bank, the Institute of Cell Biology, China National Academy of Sciences (Shanghai, China). Cells were maintained in RPMI 1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, Carlsbad, CA, USA), 100 IU/ml penicillin G sodium and 100 μg/ml streptomycin sulfate. Cultures were maintained at 37 ℃ in a humidified atmosphere of 95% air and 5% CO2.
Vitamin K2 and PC (obtained at 99% purity from soybeans) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Hepatoma cells (SMMC-7721, 104) were cultured in a 96-well plate beginning the day before the experiments. Then cells were continuously cultured in the presence of various concentrations of PC (1.25 - 400 μM) and vitamin K2 (1.25 - 500 μM) for 24-72 hours. After treatment duration, the CCK-8 assay reagent (Beyotime Institute of Biotechnology, Shanghai, China) was added to culture media and incubated for 3 hours. Absorbance was read at 450 nm on a micro-plate reader. Then, according to the best concentration and time point, synergy of the two drugs was evaluated in SMMC-7721 and other three cell lines (Hep-3B, Hep-G2 and Huh-7). The experiment was repeated in triplicate.
To identify how effective vitamin K2 and PC induce apoptosis, four HCC cell lines (104) in a 6-well plate above were treated with vitamin K2 (500 μM) combined with PC (20 μM) for 72 h, followed by assessing apoptosis by means of flow cytometric analysis. Cells were stained with FITC-annexin V (AV) and PI (BD Bioscience, Franklin Lakes, NJ, USA). Samples were run on a cytofluorimeter (Becton-Dickinson, Mountain View, CA), equipped with an argon ion and HeNe red laser to excite FITC, PE or APC, respectively, gated on the basis of side and forward scatter.
A 24-well transwell chamber (Corning, N.Y., USA) was used to evaluate the invasive ability of four HCC cell lines after vitamin K2 treatment. The upper surface of polycarbonate filters with 8 μm pores was coated with 40 μg of matrigel (Sigma-Aldrich, St. Louis, MO, USA). HCC cells were preincubated with vitamin K2 (500 μM) combined with PC (20 μM) for 72 h at 37 ℃ in a CO2 incubator and then detached and resuspended in DMEM with 1% FBS. A suspension of cells (1 × 105 cells/100 μl) was placed in the upper chambers. The lower chambers were filled with 600 μl in DMEM with 10% FBS. After 24 h of incubation at 37 ℃ under optimal conditions, the filters were fixed with 10% buffered formalin and stained with 0.1% crystal violet for 30 min. Cells that had invaded through the matrigel and reached the lower surface of the filter were quantified by counting the number of cells that migrated in five random microscopic fields per filter.
in vivo, the synergy of combination of vitamin K2 and PC was assessed in nude mice bearing HCC cells. 12 balb/c athymic (nu+/nu+) female mice, 4-6 weeks of age, were purchased from the Experimental Animal Center of the Hubei Centers for Disease Control (Wuhan, Hubei, China). The SMMC-7721 cells (107) were suspended in 100 μl PBS and were subcutaneously inoculated into the lower right flank of the nude mice (n = 6 in each group). When the tumors were 100-150 mm3 in size, group 'VK2 + PC' were treated with combination of vitamin K2 (10 mg/d/Kg) and PC (16 mg/d/Kg) intragastrically (i.g.) every 3 days throughout the experiment, while group 'control' were treated with 100 μl PBS (i.g.). In the following 36 days, the long diameter and short diameter of the established tumors were measured every 3 days. Tumor volumes during experiments were calculated according to the following equation: V (mm3) = π/6 × length (mm) × width (mm2). Differences in tumor volume were tested for statistical significance.
Total RNA of control and treated groups was isolated using Trizol (Invitrogen, Carlsbad, CA, USA) and miRNeasy mini kit (Qiagen, Germantown, MD, USA) according to manufacturer's instructions, and RNA Integrity was determined by gel electrophoresis. Then, the miRCURY™ Hy3™/Hy5™ Power labeling kit (Exiqon, Vedbaek, Denmark) was used according to the manufacturer's guideline for miRNA labelling. After stopping the labeling procedure, the Hy3TM-labeled samples were hybridized on the miRCURYTM LNA Array (v.14.0) (Exiqon, Vedbaek, Denmark). The total 25 μL mixture from Hy3TM-labeled samples with 25 μL hybridization buffer were first denatured for 2 min at 95 ℃, incubated on ice for 2 min and then hybridized to the microarray for 16-20 h at 56 ℃ in a 12-Bay Hybridization Systems (Hybridization System - Nimblegen Systems, Inc., Madison, WI, USA), which provides an active mixing action and constant incubation temperature to improve hybridization uniformity and enhance signal. Following hybridization, the slides were achieved, washed several times using Wash buffer kit (Exiqon), and finally dried by centrifugation for 5 min at 400 rpm. Then the slides were scanned using the Axon GenePix 4000B microarray scanner (Axon Instruments, Foster City, CA, USA). Scanned images were then imported into GenePix Pro 6.0 software (Axon) for grid alignment and data extraction.
Mature miRNA-specific PCR forward primers (sense DNA oligo identical to the entire mature miRNA sequences) were designed according to miRNA sequences, and the NCode™ miRNA First-Strand cDNA Synthesis Kits and qRT-PCR Kits (Invitrogen, Carlsbad, CA, USA) were used for miRNA quantitative RT-PCR analysis.
The computer-based miRNA target detection programs, TargetScan (http://www.targetscan.org/) and miRanda (http://www.microrna.org/microrna/home.do) were used to predict miRNAs binding sites in potential target mRNAs. The target mRNAs data was input into the Database for Annotation, Visualization and Integrated Discovery (DAVID, http://david.abcc.ncifcrf.gov/), which leveraged the Gene Ontology (GO) to identify the molecular function represented in the gene profile. In addition, we used the KEGG (Kyoto Encyclopedia of Genes and Genomes) database (http://www.genome.ad.jp/kegg/) and GenMAPP (v2.1) to analyze the roles of these genes in the pathways.
Cells treated with PC (20 μM), Vitamin K2 (500 μM) and combined for 72 h were lysed for 30 min in cold lysis buffer. After centrifugation at 12,000 rpm for 5 min, the supernatant was harvested as the total cellular protein extracts. The protein concentrations were determined using Bradford method. The total cellular protein extracts were separated on 8%~15% SDS-PAGE. Proteins were electro-transferred to supported nitrocellulose membranes (Pall BioTrace, USA) by a semi-dry transferor. The membranes were blocked in 5% skimmed milk in TBS-T containing 0.05% tween 20 at RT for 2 h, and then incubated at RT for 2 h with antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) to procaspase-3, procaspase-8, procaspase-9, Bcl-2, WNT3A, phospho-β-catenin and β-actin diluted in 1% skimmed milk in TBS-T, respectively, followed by incubating with the appropriate HRP-linked secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 2 h at RT. The bound antibody was visualized using an ECL system (Pierce, Rockford, IL, USA). The experiment was repeated in triplicate.
All data were the average of triplicates. The differences in mean were analyzed by Student's two-tailed t test with SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Significant differences were accepted when p < 0.05.
Vitamin K2 is the most biologically active form of vitamin K. It plays an important role in the treatment of osteoporosis and cancer [15]. A pilot study by Mizuta, et al. concluded that oral intake of vitamin K2 after curative therapy improved prognoses of patients with HCC [16]. Other reports indicated that vitamin K2 in combination with other drugs attenuated hepatic tumors in rats, and that oral administration of vitamin K2 may inhibit portal vein invasion by HCC in patients [17-19]. On the other hand, Munder, et al. [20] also found anti-tumor effect of PC. The enhancement of cancer cell apoptosis by PC anticancer analogues has been observed [21], but the effects of PC on hepatic tumors have not been reported. In this study we examined the effects of combination administration of vitamin K2 and PC on hepatic tumors in vitro and in vivo, and then inferred the mechanism preliminarily by high throughput sequencing.
In vitro, using the CCK-8 assay we showed that viability descended in a dose-dependent manner by vitamin K2 and PC in SMMC-7721 cell line (Figure 1a and Figure 1b). The maximum inhibition rate was at 500 μM for vitamin K2 and 20 μM for PC. Accordingly, the significant synergistic effect was shown in Figure 1c (p < 0.05). At 72 h, viability of cells was less than 50% in the group (VK2+PC) (Figure 1d). The same anti-tumor effect was found in the other three HCC cell lines (Hep-3B, Hep-G2 and HuH-7) (Figure 1e). Then, using apoptosis assay we showed that combination of vitamin K2 (500 μM) and PC (20 μM) could significantly induce apoptosis. The apoptosis rate was more than 50% in each group (Figure 1f). At the same time invasion assay was performed to explore the invasion activity in the presence of vitamin K2 (500 μM) combined with PC (20 μM). As shown in Figure 2a and Figure 2b, the ratio of four HCC cell lines (Hep-3B, Hep-G2, HuH-7 and SMMC-7721) penetrating the matrigel-coated polycarbonate filters reduced by 2.9, 2.7, 2.8 and 4.6 folds respectively (p < 0.05).
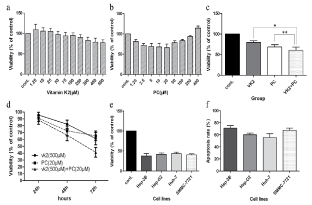 Figure 1: HCC growth inhibition effects with vitamin K2 and PC in vitro. a) SMMC-7721 cells were cultured for 48 h with concentrations of vitamin K2 indicated as follows. On the abscissa, "cont." designates the control without vitamin K2. Viability descended in a dose-dependent manner by vitamin K2; b) SMMC-7721 cells were cultured for 48 h with concentrations of PC indicated as follows. On the abscissa, "cont." designates the control without PC. Viability descended in a curvilinear dose-dependent manner by PC; c) SMMC-7721 cells were cultured for 48 h with vitamin K2 (500 μM) and/or PC (20 μM). On the abscissa, "cont." designates the control without vitamin K2 or PC. Viability descended significantly by combined administration; d) SMMC-7721 cells were cultured for 24 h, 48 h and 72 h with combined administration. Viability descended in a time-dependent manner; e) The growth inhibition effect repeated in four HCC cell lines with combined administration for 72 h. f) The apoptosis ratio of four HCC cell lines was detected by means of flow cytometric analysis.
View Figure 1
Figure 1: HCC growth inhibition effects with vitamin K2 and PC in vitro. a) SMMC-7721 cells were cultured for 48 h with concentrations of vitamin K2 indicated as follows. On the abscissa, "cont." designates the control without vitamin K2. Viability descended in a dose-dependent manner by vitamin K2; b) SMMC-7721 cells were cultured for 48 h with concentrations of PC indicated as follows. On the abscissa, "cont." designates the control without PC. Viability descended in a curvilinear dose-dependent manner by PC; c) SMMC-7721 cells were cultured for 48 h with vitamin K2 (500 μM) and/or PC (20 μM). On the abscissa, "cont." designates the control without vitamin K2 or PC. Viability descended significantly by combined administration; d) SMMC-7721 cells were cultured for 24 h, 48 h and 72 h with combined administration. Viability descended in a time-dependent manner; e) The growth inhibition effect repeated in four HCC cell lines with combined administration for 72 h. f) The apoptosis ratio of four HCC cell lines was detected by means of flow cytometric analysis.
View Figure 1
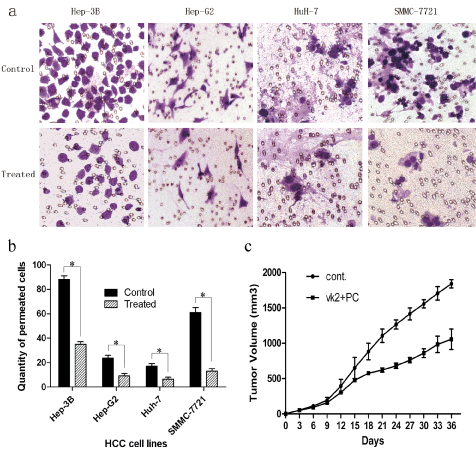 Figure 2: Invasion inhibition and findings in vivo. a,b) Four HCC cell lines were preincubated with vitamin K2 (500 μM) combined with PC (20 μM) for 72 h. The ratio of cells penetrating the Matrigel-coated polycarbonate filters were reduced by 2.9, 2.7, 2.8 and 4.6 folds respectively. Invasive ability descended signicicantly (*p < 0.05); c) Combination of two drugs was given by intragastric administration in nude mice bearing HCC xenografts. The volume of tumor in 'VK2 + PC' began to descend significantly 15 days after transplantation.
View Figure 2
Figure 2: Invasion inhibition and findings in vivo. a,b) Four HCC cell lines were preincubated with vitamin K2 (500 μM) combined with PC (20 μM) for 72 h. The ratio of cells penetrating the Matrigel-coated polycarbonate filters were reduced by 2.9, 2.7, 2.8 and 4.6 folds respectively. Invasive ability descended signicicantly (*p < 0.05); c) Combination of two drugs was given by intragastric administration in nude mice bearing HCC xenografts. The volume of tumor in 'VK2 + PC' began to descend significantly 15 days after transplantation.
View Figure 2
in vivo, tumor growth curves were depicted to compare the difference of the anti-tumor effect during the course of the experiments. As shown in Figure 2c, in both control group and 'VK2 + PC' group, tumors grew progressively and reached 150 mm3 within 9 days. Since then, untreated cell grew aggressively; however, the growth of 'VK2 + PC' treated cells were significantly suppressed (p < 0.05). At the end of 36 days, the average volume of tumor in 'VK2 + PC' group was significantly less than that in untreated group (913 ± 19 mm3 VS 1782 ± 32 mm3, p < 0.01). Sakakima, et al. [22] also found that supplementation with PC plus vitamin K2 could prevent hepatocarcinogenesis with different animal model. The two studies could complement each other and make sure the synergy of vitamin K2 and PC.
In our study, a large number of miRNAs were detected differentially expressed in the 'VK2 + PC' group. Among the 65 miRNAs that were up- or down-regulated over 2 folds change, miR-16 was the most significant (p < 0.001) (Table 1). MiR-16 was identified as potential cancer genes in the pathogenesis of chronic lymphocytic leukemia (CLL) [23]. While a miRNA gene could have several targets, Boci and Aqeilan reported that the miR-15a and miR-16-1 cluster targets Bcl-2, CCND1 (encodingcyclinD1) and WNT3A mRNA, which promoted several prostate tumorigenic features, including survival, proliferation and invasion. Together, these data suggest that miR-16 plays an important role in anti-tumor treatment [24,25].
Table 1: Combination of vitamin K2 and PC induced-miRNAs identified by microarray analysis. View Table 1
To further explore the mechanism of drug action, we used bioinformatics methods to analysis the microarray data. Target genes were predicted for all differentially expressed miRNAs and then the gene ontology (GO) analysis was performed to identify the genes function [26]. The results revealed that the antitumor effect was produced by miRNAs up-regulated, especially via the biological process (52.83%). The differentially expressed genes were most relevant to regulation of transcription (p = 9.86E-13). At last pathway analysis disclosed that differentially expressed genes were most relevant to the change of WNT signaling pathway (p = 5.26E-08) (data not shown).
As miR-16 and WNT signaling pathway might play important roles in the tumor treatment process, key proteins were detected to identify the correlation. In Figure 3a, procaspase-3 and procaspase-9 were more cleaved (p < 0.05) in group 'VK2 + PC', while procaspase-8 changed unsignificantly (p > 0.05). According to the result, mitochondrion-mediated pathway was activated, corresponding with the functional mechanism of caspase family [27]. Bcl-2, an important regulatory protein in mitochondrion-mediated pathway, was also down-regulated by the treatment [28]. On the other hand, WNT3A and phospho-β-catenin were detected. WNT signaling pathway is static in adult liver. The steady state is maintained by phosphorylation and dephosphorylation of β-catenin [29]. It was reported that activation of WNT signaling pathway could lead to liver cancer [30]. In the present study, combination administration of vitamin K2 and PC down-regulated WNT3A and phospho-β-catenin, as well as activity of WNT signaling pathway (Figure 3b).
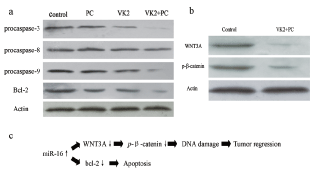 Figure 3: The related proteins activity detected by western blot analysis. SMMC cells were cultured with vitamin K2 (500 μM) and/or PC (20 μM) for 72 h. a) Proteins related to intrinsic apoptosis pathway were activated significantly; b) The activity of WNT3A and phospho-β-catenin descended in the 'VK2 + PC' group; c) A model miR-16-mediated regulation of intrinsic apoptosis pathway and WNT signaling pathway.
View Figure 3
Figure 3: The related proteins activity detected by western blot analysis. SMMC cells were cultured with vitamin K2 (500 μM) and/or PC (20 μM) for 72 h. a) Proteins related to intrinsic apoptosis pathway were activated significantly; b) The activity of WNT3A and phospho-β-catenin descended in the 'VK2 + PC' group; c) A model miR-16-mediated regulation of intrinsic apoptosis pathway and WNT signaling pathway.
View Figure 3
As mentioned above, we inferred, not concluded, combined administration of vitamin K2 and PC may inhibit hepatocarcinogenesis via up-regulating miR-16, which correlated with mitochondrion-mediated apoptosis pathway and WNT signaling pathway (Figure 3c). In addition, this study was preliminary. We will further set up a positive control group to identify the direct effect induced by miR-16 and we also speculate that more crosstalk would be found between the two pathways and further studies would be needed to identify the relationship between miR-16 and WNT signaling pathway.
In conclusion, combined administration of vitamin K2 and PC could inhibit hepatocarcinogenesis more effectively in vitro and in vivo. Moreover, up-regulation of miR-16 might play an important role in the anti-tumor progress by activating intrinsic apoptosis pathway and WNT signaling pathway.
The authors wish to thank Shanghai SensiChip Tech & infor Company (Shanghai, China) for the assistance in bioinformatics analysis.