Diagnosis of filariasis is important for Programme Managers and Physicians. This study was undertaken to determine the prevalence of lymphatic filariasis in eleven communities of Northern Taraba State. The study employed standard parasitological techniques for diagnosis, and the use of Immunochromatographic Card Test (ICT) to detect Circulating Filarial Antigen (CFA). Chi Square was used to compare and test for differences in infections among communities. 294 persons were examined, and 31.29% were confirmed positive for Wuchereria bancrofti. The performance of ICT card compared with Thick Blood Film microscopy was commendable. Thick Blood Film/microscopy identified 26.19% infection of the population, while the ICT card detected 31.29% CFA from the same population. The prevalence of CFA differ significantly (p < 0.05), and no significant difference between antigenaemia in males and females. However, chi-square test revealed a significant difference in the prevalence of antigenaemia among the different age groups. Antigenaemia was detected in 92 persons, and 34 (36.96%) persons are both antigenaemia positive and microfilaria positive, while 58 persons (63.04%) with antigenaemia but without microfilaraemia. Of the 202 negative subjects 43 (21.29%) had microfilaria in their blood samples, while 159 (78.71%) were negative for microfilaraemia. The ability of the ICT to accurately identify all those with the disease was 44.16%, while the ability of the ICT to sort out those without disease was 73.27%. The Positive Predictive Value was 36.96%, while the Negative predictive value was 78.71% burden of LF. In conclusion, since the ICT had identified 31.29% people with LF as against 26.19% by Microscopy, it was suggested that it could be employed in mass survey for Lymphatic filariasis to institute intervention activities in the area.
Bancroftian filariasis, ICT, Thick blood film, Sensitivity, Specificity
More than 100 million individuals worldwide are estimated to suffer from lymphatic filariasis (LF) caused by infection with the mosquito-borne filarial nematode, Wuchereria bancrofti [1,2]. Available literatures on the status of the disease in Nigeria show that the disease is prevalent and widespread in the six geo-political zones of the country [3,4]. Nigeria is the third most endemic country in the world for LF after India and Indonesia [5,6]. WHO [7] identified LF as the second leading cause of permanent and long-term disability and a major contributor to poverty in the world. In Benue State, Nigeria, rapid epidemiological and social-cultural assessment lymphatic filariasis amongst the Igede ethnic group was conducted [8].
Diagnosis of filariasis is important for programme managers for situation analysis, for monitoring and evaluation of intervention measures and for physicians in case detection and treatment/management [9]. However, diagnosis of bancroftian filariasis requires night time parasitological examination because the microfilariae appear in peripheral blood highest in number at night and few or none during day time [10]. During the day, the microfilariae are in the lungs; at least two circadian rhythms are involved including, one in the microfilaria and the other, a property of the physiologic rhythm of the host [11]. Usually, thick blood samples are prepared from the night blood samples collected, allowed to dry overnight or air dried could be stained Geimsa or haematoxylin. The stains allow morphological identification of filarial species. The diagnosis of this nocturnal periodic parasite is faced with several constraints including the belief that only witches take blood at night and the sacrosanct regard about it is a major setback in the diagnosis of bancroftian filariasis in Nigeria. In addition, women in Northern Nigeria are not permitted to leave their household at night, thus putting constraints on accurate diagnosis and reporting. These constraints have led to increasing quest for the most acceptable methods of diagnosis.
The use of ICT has been recommended as a rapid tool for defining the prevalence and distribution of filariasis as part of the global programme to eliminate lymphatic filariasis [12]. Galvaz Tan [13] stated that lymphatic filariasis mapping using ICT and community reporting of hydrocoel and elephantiasis increase access of the poor to diagnostic services and is a means of poverty alleviation. Gyapong, et al. [14] in a multi-country study (Benin, Burkina Faso, Ghana and Togo) employed the rapid geographical assessment of filariasis in Africa (RAGFIL) using the ICT tests. Therefore, as now the reliability of the use of immunochromatographic card test to identify infected persons with bancroftian filariasis in Savannah ecological zones need to be understood.
It was in the light of this that this study was designed to determine the performance of immunochromatographic card test status of the disease in Northern Taraba State with the view of enriching the epidemiological baseline data in Nigeria.
The study was performed in Taraba State. It lies approximately between Latitude 6025' and 9030'N and Longitude 9030' and 11045'E. It is bordered on the West by Nassarawa and Plateau States, South East by Bauchi and Gombe States, North East by Adamawa State and South west by Benue State. Taraba State is bordered to the East by the Federal Republic of Cameroon which is an international boundary. The State has a land area of about 60,291 sq.km, with a total population of 2,300,736 persons based on the 2006 National Census Figures. About 43,453 km2 of Taraba State is plain land, while 12,929 km2 is highland. The remaining 3,909 km2 is wetland. Majority of the people in the State are living in rural settlements consisting of hamlets and villages. Most of the inhabitants also live in rural agricultural areas with farming as the major occupation. Parts of the State have numerous streams traversing villages/communities and draining into the major river Benue. Communities rely mainly on the streams and rivers for water supply. Domestic water is usually stored in and around homes in drums, clay pots and all sorts of metal and plastic containers which provide permanent breeding sites for mosquitoes and ecological associates [15].
The study received ethical clearance certificate from an Institutional Health Research Committee and ethical approval of Taraba State Ministry of Health. Also, additional permission were sought and obtained from Local Government chairmen, Primary Health Care (PHC) department, Districts heads, Village heads, and key informants before the study commenced.
On the scheduled day, informed oral consent of individuals who gathered at the agreed venue (village head compound, school or church premises) were sought and obtained after the explanation of the procedures and the benefits of the study before they were examined in secrecy for chronic clinical signs and symptoms of filariasis by the criteria of Nwoke, et al. [15] and Edungbola, et al. [16]. Clinical symptoms, lymphoedema of limbs, breast and scrotal elephantiasis were recorded in personal data form containing the patients' name. Female examinations were restricted to the legs, arms and breast because of cultural inhibitions in most communities.
Night blood samples of consenting individuals were obtained between 20.00 hrs and 01.00 hrs. At each blood collection, the left thumb finger was cleaned with methylated spirit soaked in cotton wool. A sterile blood lancet was used to prick the finger and 60 μl of blood collected on a slide was used to make a thick blood film which was air-dried and stained with 10% Giemsa solution for 10 minutes [17]. The slides were then examined under a light microscope at × 10, × 40 and × 100 objective lenses. Sheathed microfilariae without caudal nuclei were classified as Wuchereria bancrofti [17].
The collection of blood was carried out between 8:00 am and 6:00 pm in the day time at a designated venue agreed upon by members of the community. The finger prick method described by Cheesbrough [17] was employed. The patient's left index finger was cleaned with methylated spirit and then punctured using a sterile lancet. The pipette dropper enclosed in the ICT kit was used to suck 60 μl of blood and applied into the sample well in the device (cassette) making sure that air bubbles were removed. Thereafter a drop of sample diluents contained in the kit was added immediately and the specimen migrated by capillary action across the cassette. The test was read as positive if the test band/line and 'C' band/line were seen in the viewing window. Any visible line on the test area indicated a positive test result even if it was lighter or darker than the 'C' line. The test was regarded as negative if only the 'C' line was seen. Positive results were apparent after 1-5 minutes. To ensure that no positive sample was deemed negative if it indicated negative after 10 minutes from the time the diluents was applied.
Data obtained was subjected to Chi Square analysis to compare and test for differences in infections among communities, using statistical package Epi- info software version 7.0. The diagnostic performance of immunochromatographic card test was assessed by calculating sensitivity, specificity, positive and negative predictive values using the formula of Chocrane [18]. The calculation was rechecked using Graph Prism 5.04 for confidence intervals as follows:
Where: TP = True positive
FN = False negative
Where: TN = True negative
FP = False positive
Of the 294 person examined 31.29% of the subjects were positive for W. bancrofti circulating filarial antigen (Table 1). The circulating filarial antigen prevalence among the communities differ significantly (p < 0.05) with no significant difference (in infection) between antigenaemia positive males and female (χ2 = 0.491, df1, p > 0.05) (Figure 1). However, Chi-square analysis showed a significant difference in the prevalence of antigenaemia among the different age group (χ2 = 11.424, df5, p < 0.05).
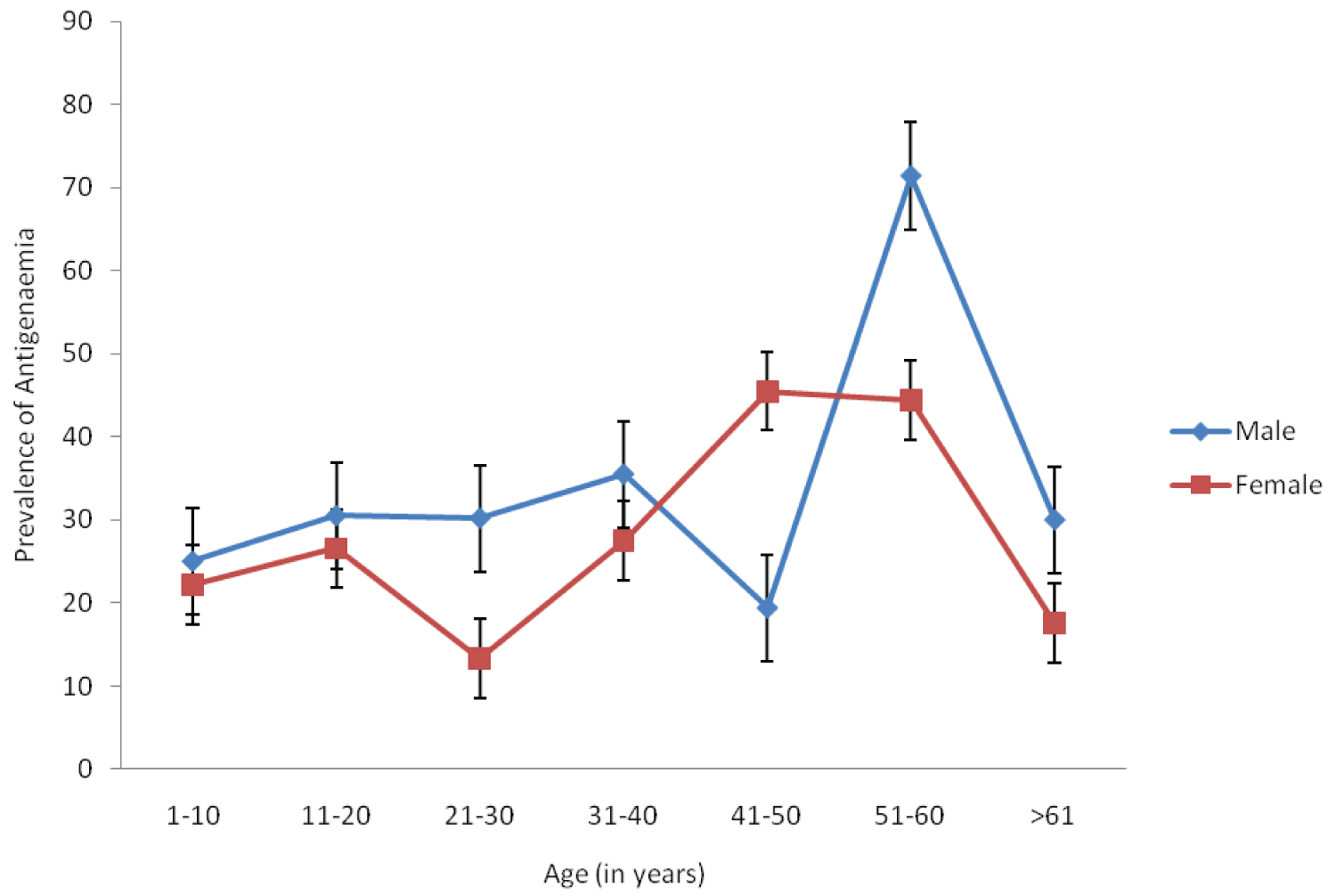 Figure 1: Prevalence of antigenaemia according to age and sex in the study area.
View Figure 1
Figure 1: Prevalence of antigenaemia according to age and sex in the study area.
View Figure 1
Table 1: Distribution of W. bancrofti antigenaemia in 11 communities in the study area. View Table 1
Table 2 compares the use of ICT test as rapid test to the true disease status as determine by microscopy and validation of ICT card as a screening test for detection of W. bancrofti in the study area. The result showed that 92 persons were detected with antigenaemia. Out of the number detected, 34 (36.96%) persons were both antigenaemia positive and microfilaria positive (True positive) and 58 persons (63.04) with antigenaemia but without microfilaraemia (False Negative). The number detected negative for antigenaemia was 202, out of which 43 (21.29%) had microfilaria in their blood samples (False positive) while 159 (78.71%) of them are negative for microfilaraemia (True negative). The ability of the ICT to accurately identify all those with the disease (i.e. sensitivity) was 44.16% while the ability of the ICT to sort out all those without the disease (i.e. specificity) was 73.27%. The Positive Predictive Value was 36.96% while the Negative Predictive Value was 78.71% burden of LF (Table 2).
Table 2: Comparison of performance of ICT as rapid screening test of LF and the true status of disease as determined by Thick Blood Film/Microscopy. View Table 2
A sound knowledge of the distribution of any disease is a pre-requisite to planning effective control measures of that disease. This study provides reliable estimates of the prevalence and burden of lymphatic filariasis in parts of Muri Emirate council. The overall prevalence of filarial antigenaemia of 31.29% among individuals in the Northern Taraba communities even after at least one cycle of MDA in some of the communities has further confirmed that bancroftian filariasis is widely distributed in Taraba State. The high level of antigenaemmia observed here is comparable to that of Braga, et al. [19] in Brazil, and Iqbal and Sher [20] in Kuwait. However, the prevalence of CFA in the present study was much higher than that of 6.45% observed by Targema, et al. [21] in Benue State of Nigeria and 21.13% recorded in Afikpo North Ebonyi State Eastern Nigeria by Ngele and Adewale [22]. The high endemicity in these communities may be as a result of the availability of favourable mosquito breeding sites and deteriorating sanitary conditions [23]. In the current study, the prevalence of circulating filarial antigen was highest among subjects aged between 50-60 years. This is consistent with the observation of Ngele and Adewale [22] who recorded same among age group of 58-62. This however, contrasts the finding of Targema, et al. [21] who recorded higher antigenaemia among the 40-49 years subjects. The ICT procedure was easy to use in the field and also very specific in detecting Circulating Filarial Antigen (CFA). It is indeed a great advancement in the diagnosis of bancroftian filariasis as compared to thick blood film preparation which is not convenient because of night blood collection [24,25].
The performance of immunochromatographic card has been evaluated in many temperate ecological zones [19,26,27]. However, in this study, 63.0% of person that were negative by the thick Blood film method seems to be positive in the ICT card test in the endemic areas. This is consistent with the findings of Braga, et al. [19] and Iqbal and Sher [20], who stated that the ICT card test, in addition to being able to detect microfilaraemia infection, probably identifies early stages of infections and single sex infections. Another possibility is that a low level of adult worm infection produces antigenaemia but no detectable microfilaraemia [6,28]. There is also the possibility since the evaluation of the test in the non-endemic area revealed a considerable number of examinations showing faint lines that following manufacturer's guide line were interpreted positive.
On the other hand, 21.2% individuals were positive by the thick blood smear appeared to be negative in the ICT card test. These findings raised concern about the specificity of the ICT whole blood card test or suggest a decrease sensitivity of the whole blood card test in cases with low microfilaraemia density. The low sensitivity of the ICT was reported previously from India in cases with low mf density [29]. Similarly, Koyandun, et al. [30] reported a significant decrease in Circulating Filarial Antigen (CFA) at 18 months after mass drug administration among the Myanmar migrants in Thailand. This might be the possible explanation to the above observations in the study area.
The performance of ICT card in comparison to Thick Blood Film microscopy in the present study is commendable. Thick Blood Film/microscopy identified 26.19% infection of the population, the ICT card detected CFA from 31.29% of the same population. This is consistent with the findings of Braga, et al. [19] who observed higher antigenaemia in comparison to microfilaraemia in Brazil. The 44.16% sensitivity and 73.27% of specificity recorded in this study is higher than the low sensitivity reported by Sunish, et al. [29] in India. However, the sensitivity observed in this study is lower than the 93.8% sensitivity recorded by Iqbal and Sher [20] among migrant workers in Kuwait. Since the card requires minimal laboratory and technical support compared to microscopy and yield results in few minutes on the spot, it is thus more suitable for diagnosis of filarial infection in clinical laboratories and field survey.
The use of ICT card for mapping endemic communities has been recommended by Brooker and Utzinger [31] in order to target Mass Drug Administration. However, large scale field use of ICT card test in control programmes in endemic communities will require assessment of its cost and suitability. A cost analysis of the ICT card test and Thick Blood Smear Method were carried out in Sri Lanka [19]. The thick Blood Smear method was shown to have a lower price [ICT cost per unit US$ 2.75 while TBS cost per unit - US$ 0.30] [26]. However, certain features in the ICT card test proved to be extremely advantageous; high sensitivity, the ability to offer prompt diagnosis, no need for specialized technicians. These combined characteristics overcame the lowest price of thick blood smear method, making to be overall more cost effective option thereby justifying its use as a diagnostic tool in screening in endemic areas [19].
In conclusion, the use of ICT had identified 31.29% while Microscopy identified 26.19% of the same population with the disease. Thus the use of ICT as a diagnostic tool could be employed in a mass survey for lymphatic filariasis. This study has provided a rapid analysis of the situation of the disease in the study area, hence urgent steps need to be taken to implement lymphatic filariasis control programme in the entire Taraba State.
The authors declare that there is no conflict of interest regarding the publication of this paper.
This study received full sponsorship from Taraba State University, Jalingo through the TETFUND grant. We appreciate and thank the Chairmen and Directors Primary Health Care of Muri Emirate council, Commissioner and Permanent Secretary Ministry of Health, Taraba State for collaborating in carrying out this research project.