The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a coronavirus strain, and its main way to get access to the cells is via the angiotensin converting enzyme 2 (ACE2) and causes the Coronavirus Disease 2019 (COVID-19). ACE2 is an important enzyme in the pathogeneses of hypertension. For the treatment of hypertension, it is commonly used angiotensin II receptor blockers (ARBs) and angiotensin II converting enzyme inhibitors (ACEIs). It is clear that there is a lot of uncertainty and controversy regarding SARS-CoV-2 and its interaction with ACE2. These issues should be solved with more studies. Thus, the use of medications such as ACEIs and ARBs should be individualized and in the majority of times its' suspension is not necessary.
SARS-COV-2, Covid-19, Renin-angiotensin-aldosterone system, Angiotensin-converting enzyme inhibitors, Angiotensin receptor blockers
SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; COVID-19: Coronavirus Disease 2019; SARS-CoV: Severe Acute Respiratory Syndrome Coronavirus; WHO: World Health Organization; ARBs: Angiotensin II Receptor Blockers; ACEIs: Angiotensin II Converting Enzyme Inhibitors; ACE1: Angiotensin-Converting Enzyme 1; ACE2: Angiotensin-Converting Enzyme 2; RAAS: Renin-angiotensin-aldosterone system
The SARS-CoV-2 outbreak started at Wuhan, China and in January of 2020, World Health Organization (WHO) declared global pandemic [1]. SARS-CoV-2 is a CoV-2 with similarities when compared its genomic and proteomic with SARS-CoV. Furthermore, SARS-CoV-2 and SARS-CoV main way to get access to the cells is via the angiotensin converting enzyme 2 (ACE2) [1-3]. Since there is no treatment, or vaccination to this specific etiological agent it resulted in a global shift in the researchers' objectives. This study addressed the relation of SARS-CoV-2 and Renin-Angiotensin-Aldosterone System and its influence on hypertension and cardiovascular system. The research was made in Scielo and PubMed websites with the following key words: "SARS-CoV-2", "ACE2", "Heart", "Renin-angiotensin-aldosterone system" and "COVID-19". The process of article selection followed the research with the key words, was the screening process where we took in consideration studies without conflict of interest, in English and Portuguese languages and not older than 10 years. However, two articles, regarding RAAS, older than 10 years were kept due to their quality and relevance.
RAAS is responsible for several physiological pathways, specially the blood pressure homeostasis. The first element of this system is renin [4]. The majority of renin in the body is found in the juxtaglomerular cells of the kidney [4]. Renin cleaves angiotensinogen, which results in the production of angiotensin I. Another molecule in RAAS is angiotensin II, this is formed by angiotensin I cleavage by ACE. There are two main receptors for angiotensin II (AT1 and AT2), which are differentially expressed on the cell surface [5-7].
AT1 receptors are bound to G proteins, which mediates signaling pathways, such as activation of phospholipase C [6]. These can lead to vasoconstriction, profibrotic action, growth stimulation and aldosterone release. Aldosterone increases sodium retention, thereby increasing blood pressure. The interaction of AT2 receptors and G proteins is controversial. AT2 receptor mediated signaling may antagonize AT1-mediated signal transductions [7]. However, accumulating evidence indicates that AT2 receptor-mediated signaling also mediates the detrimental action of angiotensin II [7].
Another RAAS-derived bioactive molecule is angiotensin-(1-7) [4]. The synthesis of angiotensin-(1-7) is mediated by a unique RAAS pathway involving ACE2, which is expressed predominantly in vascular endothelial cells of the heart and kidney [4]. Both ACE and ACE2 metabolize angiotensin I. However, the resulting peptides are different. ACE converts angiotensin I to angiotensin II, whereas ACE2 cleaves angiotensin I into angiotensin 1-9. Although angiotensin 1-9 itself exhibits no known biologic activity, angiotensin 1-9 is cleaved by ACE, and bioactive angiotensin-(1-7) is synthesized [4]. ACE2 can also directly cleave angiotensin II to form angiotensin-(1-7). Angiotensin (1-7), when it binds to its receptor, MAS, performs several beneficial actions, such as antihypertensive, anti-inflammatory, anti-proliferative and anti-fibrotic. The counterregulatory axis of the RAAS thus constitutes: ACE2/Angiotensin (1-7)/MAS receptor [8].
Angiotensin-(1-7) plays a role as an antihypertensive molecule through the stimulation of the release of vasodilator prostaglandins and nitric oxides and inhibition of angiotensin II [4]. On the lungs, MAS receptor is expressed in smooth epithelial tissue and in the air ways, alveolar, smooth vascular muscle and endothelial cells [8-11].
The MAS receptor has also been identified in immune cells, such as dendritic cells, lymphocytes, macrophages, eosinophils, neutrophils and alveolar macrophages, indicating a cellular mechanism for angiotensin (1-7) actions in the immune system [8,9,12,13].
Studies utilizing MAS receptor deficient mice have indicated that the interaction between angiotensin-(1-7) and the MAS receptor plays vital roles in endothelial function, aortic relaxation, and heart function [4] (Figure 1).
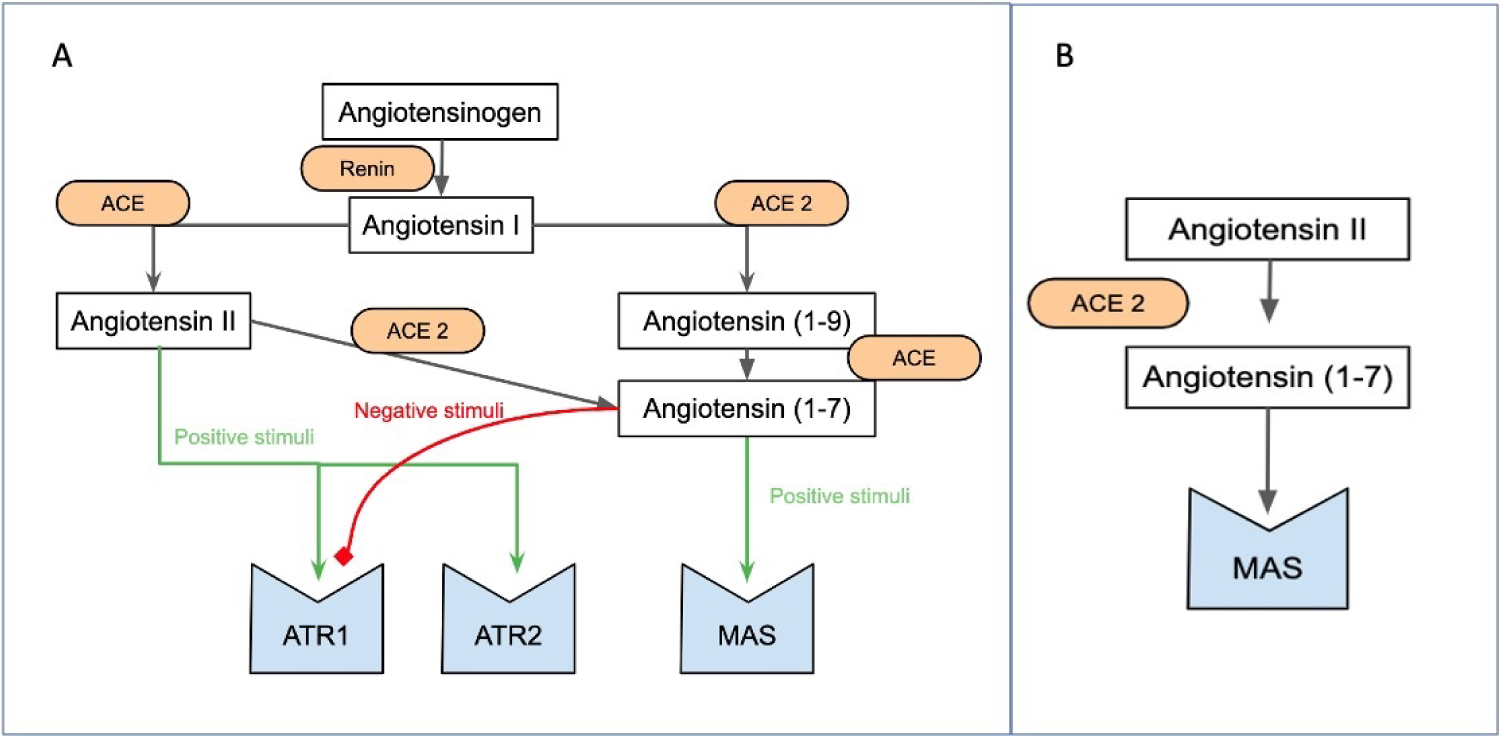 Figure 1: Schematic representation of the RAAS (partially described). (A) Interactions of ACE and ACE2; (B) Demonstrates direct action of ACE 2 in angiotensin II.
View Figure 1
Figure 1: Schematic representation of the RAAS (partially described). (A) Interactions of ACE and ACE2; (B) Demonstrates direct action of ACE 2 in angiotensin II.
View Figure 1
Over 600 million people have high blood pressure [14]. In order to treat this disease, it is commonly used angiotensin II converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs).
Studies by Ferrario, et al. have shown that ARBs (losartan) and ACEIs (lisinopril) increases, respectively, the expression of ACE2 by 4.7 and 2.8 times in rats when blood pressure decreases [15]. This increase was found in heart and kidney cells [1]; it is necessary new studies to see whether or not if this affirmation is also valid in pulmonary cells. ACEIs are known to reduce concentrations of angiotensin II, substrate for ACE2, making the ACE2 more available to virus infection [1]. Lower expressions of this enzyme cause inflammation and lung edemas [16].
A possible new approach to decrease the blood pressure is the use of direct renin inhibitors (DRIs). It has been shown that DRIs diminish the activity of the RAAS and also downregulate ACE2 in animal models, subsequently lowering the infection potentials [17]. Thereby with DRIs, cardio-pulmonary protection is maintained due to the inhibition of angiotensin I and II. Even with these new approaches, it is important to take in consideration that the decrease of ACE2 can cause inflammation and lung edema [16]. It is necessary to make an individual assessment of every case.
Some studies suggest that cardiovascular injuries secondary to the virus could be related to ACE2 [17,18]. Hence, ACE2 regulates negatively angiotensin II, having a protective role against respiratory insufficiency and its progression [17]. SARS-CoV-2 contains four main structural proteins: nucleocapsid protein (N), spike protein (S), protein envelope (E) and membrane protein (M) [19]. The virus binds via the spike protein to the ACE2 receptor and, through this link, enters the host cell, where ACE2 inactivation occurs, which favors lung injury [18]. As ACE2 has high concentrations in the heart, potentially serious damage to the cardiovascular system can occur [20].
Patients with preexisting cardiovascular diseases seem to have higher ACE2 serum levels, which could lead to more harsh manifestations of COVID-19 [20-22]. Likewise, individuals with high blood pressure express a higher ACE2 concentration due to ACEIs or ARBs usage, which could increase the chances of infection [23]. It is necessary to stress that even with these results, we have to be careful, since the number of individuals tested was low, and the majority of them were old [18].
A lot of the physiological and biochemical mechanisms of COVID-19 are still uncertain; however we can already have some intake into interesting paradoxical events that are found in SARS-Cov-2 patients. One of the main topics of controversy is the fact that the disease tends to infect more and be deadlier in the elderly and in patients with comorbidities [23]. It is interesting that in Cheng study [24], the older the person is, the lower the expression of ACE2 is found, which would theoretically be less prone to infection. It is relevant to show that hypertension patients naturally have higher expression of ACE2.
An important finding described by Vaduganathan [25] is that the level of circulating ACE2 is usually low, and its main function is being performed mainly in the lungs of healthy individuals, which would make it harder to infect individuals. The idea that the higher the concentration of ACE2, the higher is the chances of contracting the virus is overthrown by Vaduganathan [25] study. This study states that 35% of patients who die by COVID-19 showed the presence of viral RNA, which in turn was associated with reduced ACE2 protein expression.
Considering the disease's spread, lethality and treatment as easy tasks to take care of is extremely naive, since all the data collection was done in a short period of time, giving us a shorter time to evaluate, comprehend and develop around these findings. It is clear that more investment and effort are needed to develop scientifically proven medications and vaccines [17].
The treatment with ACEIs and ARBs has been shown that it can up-regulate ACE 2 receptor expression and it is thereby theoretically possible that pre-existing use of these drugs might predispose a person to infection with a greater viral load of SARS-Cov-2 as a result of greater ACE 2 expression [26].
Although there are no randomized clinical trials or meta-analysis on the topic, the first published observational studies converge on a safe use of medications that work in the RAAS under COVID-19. In the largest observational study with 12,500 patients tested positive for COVID-19 in New York, USA, previous treatment with RAAS-acting medications is not associated with worse clinical outcome, defined as ICU hospitalization, mechanical ventilation or death. Findings consistent with other types of cardiovascular medication [27].
The use of Asian, European and North American observational databases confirmed the increased risk of some comorbidities (coronary heart disease, heart failure, electrical diseases, smoking and chronic obstructive pulmonary disease) associated with elderly people over 65 years with higher inhospital mortality. But interestingly, in 8910 patients this association was not verified with the use of medications that influence RAAS, including the use of ACE inhibitors was associated with reduced mortality in this cohort (OR 0.33, 95% CI 0.20-0.54) [28].
Zhang P, et al. published a retrospective multicenter study conducted in Chinese inhospital centers use of ACEI/ARB was associated with lower risk of all-cause mortality due to COVID-19 compared with either nonuse of ACEI/ARB (adjusted HR, 0.37; 95% CI , 0.15-0.89; P = 0.03, or use of a different class of anti-hypertensive agent (adjusted HR, 0.30; 95% CI, 0.12-0.70; P = 0.01) in COVID-19 patients with hypertension, it is important to highlight possible biases associated with retrospective [29].
Mancia G, et al. published in an Italian case-control study, 6272 patients with COVID-19 were compared with 30,759 controls, in conditional logistic-regression multivariate analysis the use of ACE inhibitors or ARBs did not increase the probability of COVID-19 infection or mortality [30]. This goes against observational studies from the UK (n = 1200) and Wuhan, China (n = 1178) suggesting that chronic use of ACE inhibitors or ARBs is not associated with death or admission to intensive care in patients with COVID-19 [31,32].
The consistent findings of these cohorts help to foster the idea of continued use of these medications in patients under investigation or receiving treatment for COVID-19. However, we can utilize these findings to defend the recommendations done by several cardiology societies. The American Heart Association (AHA), Heart Failure Society of America (HFSA), Brazilian Cardiology Society (BCS), European Society of Cardiology (ESC) and American College of Cardiology (ACC) states that patients taking ACEI and ARBS who contract COVID-19 should continue treatment.
The protective effect of ACEI/ARB also may result from the favorable effect on microvascular complications; this reduces cardiovascular and renal morbidity [29]. Liu Y, et al. suggest that anti-hypertensive angiotensin II receptor blockers were associated to mitigation of disease severity in elderly COVID-19 patients [33]. Meng J, et al. suggest that renin angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension [34]. It is important to point out in these studies that we have an ethnically mixed population, as this minimizes a potential effectiveness bias for medications related to race. But always emphasizing that the answer will be by randomized clinical trials.
New patients could be treated with DRIs, since these drugs diminish the expression of ACE2 [1], but without a proven level of evidence. There is currently no effective drug for the treatment of COVID-19, and it is speculated that ACE2 spike protein-based vaccine and rhACE2 may become one of the most promising approaches, improving the prognosis of patients with this viral infection [24].
It is clear that there is still a lot of uncertainty and controversy around SARS-CoV-2 and ACE2 and only with more studies we will have proven answers. The utilization of ACE and ARBs medications should be individualized and in the majority of time its' suspension it is not necessary.
This pandemic has less severe lethality, and different spread rates when compared to the Spanish flu and Bubonic plague. However, we cannot underestimate the changes that our society, economics, relationships and education are suffering. Getting close to a new medication or even a vaccination will demand a collective effort.
The authors report no disclosures. All authors and contributors agree to the conditions outlined in the Authorship and Contributorship section of the Information for Authors. The authors have read the Journal's position on issues involved in ethical publication. There was no sponsorship for the scientific article and there was no conflict of interest with all the authors.