Schistosomiasis (Bilharziasis) is water borne parasitic disease caused by a blood fluke (trematode) of the genus Schistosoma. Adult schistosome worms live in mammalian host and the intermediate host is aquatic snail. Schistosomiasis ranks second to malaria in terms of prevalence and persistence with grave public health and socio-economic importance in endemic communities. This study assessed the prevalence of urinary schistosomiasis among students of five selected secondary schools in Ifedore Local Government of Ondo State. A total of four hundred and thirteen (413) students urine samples were examined for eggs of Schistosoma and 57 students (13.8%) excreted eggs of S. haematobium in their urine. There was no significant difference (P > 0.05) in the prevalence of urinary infection between genders. Meanwhile, the prevalence of hematuria was significantly higher (P < 0.05) in male (22.7%) than the female gender (14.4%). The highest prevalence was observed among age group 16-18 years (21.7%) while the least prevalence was observed in age group 10-12 years (11.5%). The study confirmed the prevalence of S. haematobium in the study area. Proper sanitation, water control and snail elimination as well as community-based programs are highly needed to reduce the prevalent of urinary schistosomiasis in the study area.
Schistosomiasis, Hematuria, S. haematobium
Schistosomiasis also known as bilharziasis, is waterborne disease caused by parasites of the genus Schistosoma, a digenic trematode that reside in the blood vessels of man and livestock four species of this Schistosoma are common to man; S. mansoni, S. haematobium, S. japonicum and S. intercalatum. Schistosomiasis is one of the most important neglected tropical diseases in terms of public health affecting more than 200 million people and is second only to malaria in terms of public health importance, killing an estimated 280,000 people each year in the African region alone [1]. S. haematobium is the causative agent of urinary schistosomiasis and it is most prevalent in Africa (National Travel Health Network and Centre) [2].
Urinary schistosomiasis leads to a variety of clinical manifestations as hematuria; The presence of blood cells in urine. Other associated features are dysuria and suprapubic pain. In sub-Saharan Africa, S. haematobium infection is estimated to cause 70, 32, 18 and 10 million cases of hematuria, dysuria, bladder-wall pathology and major hydronephrosis respectively [3]. Other health impacts associated with the disease are risk of anemia, bladder cancer, nutritional deficiencies, delay puberty in children, stunted growth in children and impairment of cognitive development in infected individuals as well as decreasing physical activity, school performance, work capacity and productivity [4, 5]. The highest prevalence and intensities usually found in school-age children, adolescent and young adults [6].
Several environmental and socio-economic factors have been identified to be responsible for the continued persistence of intestinal parasitic infection in children. Some of these factors include poor sanitary conditions, unhygienic practices, lack of potable water, poor housing and poverty [7,8]. The conditions lead to continued exposure to the causal parasites and thus high rates of re-infection [9]. This infection is also associated with rural agricultural and other human activities around the freshwater bodies such as swimming, fishing, washing and bathing in ponds, rivers and dams, where the snail intermediate hosts breed. The tradition of African rural women in company of their children to wash household utensils in nearby stagnant water is not left out in socio-economic factors.
School age children were thought to have frequent water contact that would make them more vulnerable to schistosomiasis, and hence this age group would be associated more frequently with schistosomiasis problems [10,11]. Schistosome infections are usually at their peak in late childhood to early adulthood. In some parts of Africa, the onset of hematuria due to urinary schistosomiasis is very common in adolescent boys, and due to lack of knowledge, it is seen as a normal phenomenon in some communities [12]. Hence, this study aimed to assess the prevalence of urinary schistosomiasis among school pupils in Ifedore Local Government Area of Ondo State, Nigeria.
A cross-sectional study was conducted among students of five (5) selected secondary schools in Ifedore Local Government Area focusing on determining the prevalence of urinary schistosomiasis in the study area.
The study was carried out among students of selected secondary schools in Ifedore Local Government Area of Ondo State. Ifedore Local Government is one of the eighteen Local Government Areas in Ondo State. The Local Government Area with headquarters in Igbara Oke is bounded to the North and East by Akure South Local Government Area to the south by Osun State, to the west by Ekiti State (Figure 1). Ondo State is located within longitude 4.89° E and latitude 6.89° N. Five secondary schools were randomly selected from the five selected towns that make up the Local Government Area. These schools are: Anglican Grammar School Igbara Oke with student population of 835; The Apostolic High School, Ilara Mokin with student population of 768; Ayo Grammar School Ipogun with a student population of 324; Anglican Grammar School Ijare with student population of 644 and Community High School Isarun with student population of 220. The major water sources in all these areas were springs, stream and rivers, which are for domestic, occupational and recreational purposes such as drinking, bathing, washing, farming and swimming.
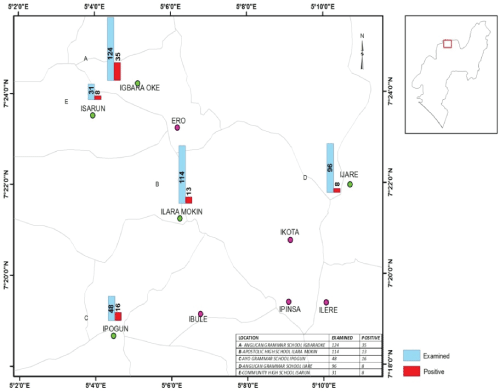 Figure 1: Map of Ifedore local government area, Ondo State, Nigeria showing the sampled towns using Geographic Information System (GIS) (2017).
View Figure 1
Figure 1: Map of Ifedore local government area, Ondo State, Nigeria showing the sampled towns using Geographic Information System (GIS) (2017).
View Figure 1
The required sample size was determined using single population proportion formula and assuming a 58.1% proportion of urinary schistosomiasis from previous study by Ekpo, et al. [13].
n = sample size
Z is the confidence level (Z = 1.96 for 95%)
E is the desired margin of error (0.05)
P = proportion of schistosomiasis in the population from previous study = 58.1% = 0.581
n = 375
Therefore with 10% attrition rate, 413 students whose age range from 10-18 years were examined from the five selected secondary schools to participate in the study.
Urine samples were collected between 09:00 and 12:00 h. Each pupil was given a clean, dry, universal bottle to urinate in with emphasis on the last drop. The urine samples collected were transported in ice packs to the Central Laboratory of Ondo State Primary Health care board, Oke Eda for analysis. Laboratory analysis of the urine was done using the centrifugation method. 10 mls of urine was centrifuged at 1,500 rpm for 5 minutes and the residue was examined under the × 10 objective of the microscope for the presence of terminal spine ova of S. haematobium. Eggs of S. heamatobium were counted under light microscope at low magnification. Results were expressed as the number of S. haematobium eggs/10 ml urine. Cases of hematuria were observed and recorded.
Data were analyzed statistically using Statistical Package for Social Sciences (SPSS) software version 20.0 at P < 0.05 level of significance. The Chi square (χ2) test was used to test possible association of infection with respect to age and sex. P-values less than 0.05 were considered statistically significant.
Before the commencement, approval was sought from the Primary Health Care Board, Oke Eda Akure, Village heads, and the principals of the schools used for the survey. Consent forms were given to the pupils to take home to their parents. Those whose parents consented were the ones recruited for the study.
In all, four hundred and thirteen (413) students from five randomly selected secondary schools in Ifedore Local Government Area of Ondo State were examined for urinary schistosomiasis and fifty seven (57) students were positive to the infection representing 13.8%. Urinary schistosomiasis infections were not significantly different between gender (p = 0.093) even though male had higher prevalence of 16.6% than female with 10.9% but these figures were not significantly different (p = 0.093) (Table 1).
Table 1: Prevalence of urinary schistosomiasis by gender in Ifedore local government area. View Table 1
Age prevalence of schistosomiasis showed that 24 students that were within the age range 13-15 years of age that participated in the study tested positive for schistosomiasis with the percentage prevalence of 16.3%. However, the highest prevalence of 21.7%, n = 5 was observed in age group 16-18 years while the least prevalence of 11.5%, n = 28 was observed in age group 10-12 years (Table 2). Meanwhile, Chi-square (χ2) analysis of the data showed that the age of the students has nothing to do with the prevalence of urinary schistosomiasis in secondary schools (p = 0.322).
Table 2: Prevalence of schistosomiasis among age groups in Ifedore local government area of Ondo State. View Table 2
The result in Table 3 showed that not all the students that manifested hematuria were positive to S. haematobium ova. For instance 48 male students had hematuria while 35 of them were positive to urinary schistosomiasis representing 22.7% because of the presence of ova with terminal spine in their urine. Similarly, of the 29 female students that had hematuria, 22 were infected with urinary schistosomiasis (Table 3). The hematuria were noted to be more among the male (22.7%) than the female gender (14.4%).
Table 3: Prevalence of Hematuria by gender. View Table 3
Table 4 showed that the prevalence of hematuria varied significantly among the age groups (P < 0.05). The highest prevalence (60.9%) of hematuria was observed in age group 16-18 years while the least prevalence (12.3%) of hematuria was observed in age group 10-12 years. Generally, it was noted that the prevalence of hematuria increases as the age of the subject increases (Table 4).
Table 4: Prevalence of Hematuria among age cohorts in Ifedore local government area. View Table 4
Result presented in Table 5 showed the prevalence of urinary schistosomiasis among the selected secondary schools. The prevalence was observed to vary significantly among the secondary schools (P < 0.05). The highest prevalence of the disease was observed in Ayo Grammar School Ipogun (33.3%) while the least prevalence (5.2%) was observed in Anglican Grammar School Ijare. In addition, 20.9%, 6.1% and 9.7% prevalence were recorded in Anglican Grammar School Igbara Oke, the Apostolic High School Igbara Oke and Community High School Isarun respectively.
Table 5: Prevalence of schistosomiasis among selected secondary schools in Ifedore local government area. View Table 5
Table 5 also showed the prevalence of urinary schistosomiasis in relations to distance from the transmission site (river). Ayo Grammar School Ipogun with the highest prevalence of 33.3% had the shortest distance (< 1.0 km) from the Ipogun river (transmission site) while Anglican Grammar School Ijare with the lowest prevalence of 6.1% had the longest distance (< 10.0 km) to the transmission site. The result showed that there was inverse relationship between prevalence of infection and distance from the river.
From 413 students examined, 57 tested positive to urinary schistosomiasis with the 13.8% prevalence. Considering the pathology of the parasites (S. haematobium) and the morbidity associated with the disease, this prevalence (13.8%) is considered to be of public health importance. Similar prevalence (11.2%) was recorded by Ekpo, et al. [13] in a study conducted among pre-school children in rural community near Abeokuta in Nigeria.
Prevalence of 13.8% is considered low by WHO [14] nonetheless infection is wide spread in the area. The low prevalence obtained was in accordance with other studies in Nigeria such as Ekejindu, et al. [15] in Anambra State (11.8%), Akogun [165] in Malumfashi, Bauchi State (17%), Istifanus, et al. [17] in Malumfashi (17%), Okoli, Odaibo [18] in Ibadan (17.4%) and Fajewonyomi, Afolabi [19] in Ile-Ife, Oyo State (20.50%).
The prevalent rate of the infection in this area suggests repeated exposure to infection by students examined as it had also been documented by Oniya and Odaibo [20]. Interaction with some students examined revealed that a number of the students still bath and drink from some of the rivers (like Apomu river and Ipogun river) in the area that had been found out to serve as reservoir for the host organism (S. haematobium) and intermediate host (Bulinus spp) of this infection [21].
It is important to note that the level of prevalence of the infection established in this study is 18.6% similar result (18.0%) had already been established in the Local government in previous study by Oniya and Olofintoye [22]. However, it is expected that the prevalence should have drastically reduced since the time of Oniya and Olofintoye [22], however this is far from what is shown by the result of the study. The reason for this might be ingenuity in effort of the government and other agencies at reducing the scourge of this infection, other reason might be due to the ignorance of the people about the mode of transmission of the disease in their communities. Except for Oniya, et al. [21] study in Ifedore Local Government Area of Ondo State, other studies in Nigeria had found out higher prevalence of schistosomiasis among their study population as compared with what was found out in this study. For instance, Odaibo, et al. [23] recorded prevalence of 30.4% in Ondo State; Akinwale, et al. [24] recorded prevalence of 54.6% in their study in Ogun State and Ugbomoiko, et al. [25] recorded prevalence of 62.0% among people in two peri-urban communities (Eko-ende and Ore) in Osun State, South Western Nigeria. Also, Ozowara, et al. [26] documented 46.18% prevalence of schistosomiasis among school children in a Local Government Area in Ebonyi State while a more recent study conducted by Singh and Muddasiru [27] among children in riverine areas of Sokoto State recorded the prevalence rate of 60.8%.
The results further showed that there was no significant difference (χ2 = 2.82; P > 0.05) in the prevalence of schistosomiasis between gender. Meanwhile the pathological effect of the parasite was more pronounced among the male subjects (22.7%) than the female subjects (14.4%). This suggests that the pathology associated with S. haematobium as presented by hematuria is a function of intensity and frequency of exposure. The male subjects are known to visit the rivers more than the female.
The results indicated that students who tested positive for schistosomiasis were also at greater risk of hematuria. This implies that the hematuria test may be a very useful diagnostic tool for the detection of Schistosoma haematobium infections. The association between Schistosoma haematobium and hematuria had also been reported by Anosike, et al. [28] and Vender Warf, et al. [3]. There are contradictory reports regard to gender prevalence as reported by Singh and Muddasiru [27], that schistosomiasis was more pronounced among male (79.59%) compared to the female population (20.41%) in Sokoto. Similarly, result of Mbata, et al. [29] in a study conducted in a Local Government Area in Benue State reported that schistosomiasis was higher among female (152 (23.13%)) than male (148 (22.52%)), this is also corroborated by report of Nkegbe [30] in Ghana.
The prevalence of schistosomiasis among the age groups showed that the disease increases as the age group increases. The confirmation that age group is one of the predisposing factors that exposed the subjects to the disease has also been reported by Abebe, et al. [31]. The prevalence among the age group 10-18 years were also supported by Singh and Muddasiru [27] who found out that the prevalence of schistosomiasis was significantly higher among children within age range 9 to 12 years of age. This study however, found out that there was linear relationship between age and prevalence of schistosomiasis infection among the students examined.
The pattern of human water contact activity was dependent on the nature, accessibility and suitability of each site. The closeness of the schools to the host stream and the frequent exposure to the stream contributed to high prevalence of urinary schistosomiasis among the students. Chidi, et al. [32] revealed that the respondents who were living closer to rivers/streams and ponds were more at risk of getting schistosomiasis than those living far. However, Imran, et al. [33] suggested that the risk of infection may not depend on proximity to the river but there could be possibility of scattered water bodies as a source of infections of schistosomiasis, that are even more suitable for harboring snails vector than the closest river. Swimming activities were observed at the closest site to the study area by the students. In general, washing limbs, fetching water, clothes and farm tools as well as farm products were found to be activities of importance, in the transmission of schistosomiasis.
Considering the prevalence observed in the study area, it showed that urinary schistosomiasis is still a disease of public health importance in Ifedore Local Government Area. Therefore, more researches focusing on vector control should be carried out in this area. Similarly, presence of hematuria among the infected students calls for mass chemotherapy in the community. However, the chemotherapy will not be effective unless the individuals stop bathing, swimming and washing in contaminated water. To achieve this, government should provide potable water such as bore holes and pipe-borne water in the community to reduce contact with the contaminated river.
Special thanks to the entire staff of Ondo State School of Nursing, selected schools and Ondo State Central Laboratory, thank you so much.