Fibromatosis or desmoid tumor is a benign tumor that rarely affects the breast. It represents 0.2% of all breast tumors and 4% of all extra-abdominal desmoid tumors. Wide local excision with adequate safety margins is considered the standard of care. We report three cases of breast fibromatosis who were presented to and operated in the sultan Qaboos University. All of these cases underwent wide local excision. After close regular follow up now for six months no local recurrence was reported.
Brest fibromatosis, Desmoid tumor, Rare breast disease
Fibromatosis or desmoid tumor is known as a benign tumor that originates mainly from the fascia or aponeuroses of the abdominal wall muscles or from the muscles of the shoulders and pelvic girdles. Fibromatosis of the breast is an unusual site for its occurrence with reported incidence of less than 0.2% of all primary breast tumors [1,2]. It may arise in the breast parenchyma or represent extension of a lesion arising deep in the aponeurosis of chest wall or shoulder girdle [3,4]. It is characterized by being locally aggressive with significant risk for local recurrence rate even with adequate surgical resection but no metastatic potential [5]. By definition, fibromatosis is non-encapsulated well-differentiated fibroblastic lesion composed of relatively uniform fibroblasts and collagen and forming a firm, solitary, or multinodular mass with an infiltrative growth pattern. It occurs mainly in women, typically between the ages of 25 and 45 years and it is extremely rare in men [6]. Despite its rarity, this condition may mimic primary breast malignancy. Fibromatosis of the breast can occur either sporadically or genetically like in familial adenomatous polyposis (FAP) and Gardner's syndrome (GS) [7]. The definite etiology is unclear; however, it was reported as a consequent of surgical trauma or silicone implant as well as being an association with Gardener's syndrome [8,9]. The common presentation is a unilateral, solitary mass that is sometimes associated with skin retraction or fixation to the underlying pectoralis major muscle. Bilateral desmoid tumors have been reported in up to 4% of patients [2], and multicentric lesions have also been found [10]. Wide local excision with adequate safety margins is considered the standard of care. We report three cases of breast fibromatosis in our surgical breast at Sultan Qaboos University Hospital.
An 18-year-old woman presented to our breast surgical clinic with a palpable right breast mass, which she had noticed a few days earlier. She denied any history of nipple discharge and skin changes. She had no family history of breast cancer. She had no other illness and no history of oral contraceptive use. There was no previous breast surgery or trauma to the breast or chest wall. Clinical breast examination revealed ill-defined mass at 9 o'clock along the right mid-axillary line. It was hard in consistency, fixed and mobile. It measures 2 × 2 cm in size. The axillary lymph nodes were not palpable. The sonographic evaluation of the mass revealed an irregular hypoechoic mass with speculated margins and associated with mixed posterior acoustic features. There was no internal vascularity. It measured 2.7 × 1.7 × 4 cm (Figure 1). The axillary lymph nodes were sonographically normal. The ultrasound findings were suspicious for malignancy and were characterized, according to the Breast Imaging Reporting and Data System (BI-RADS), as BI-RADS 4C. This was followed with ultrasound guided True cut biopsy that showed benign myofibroblastic lesion.
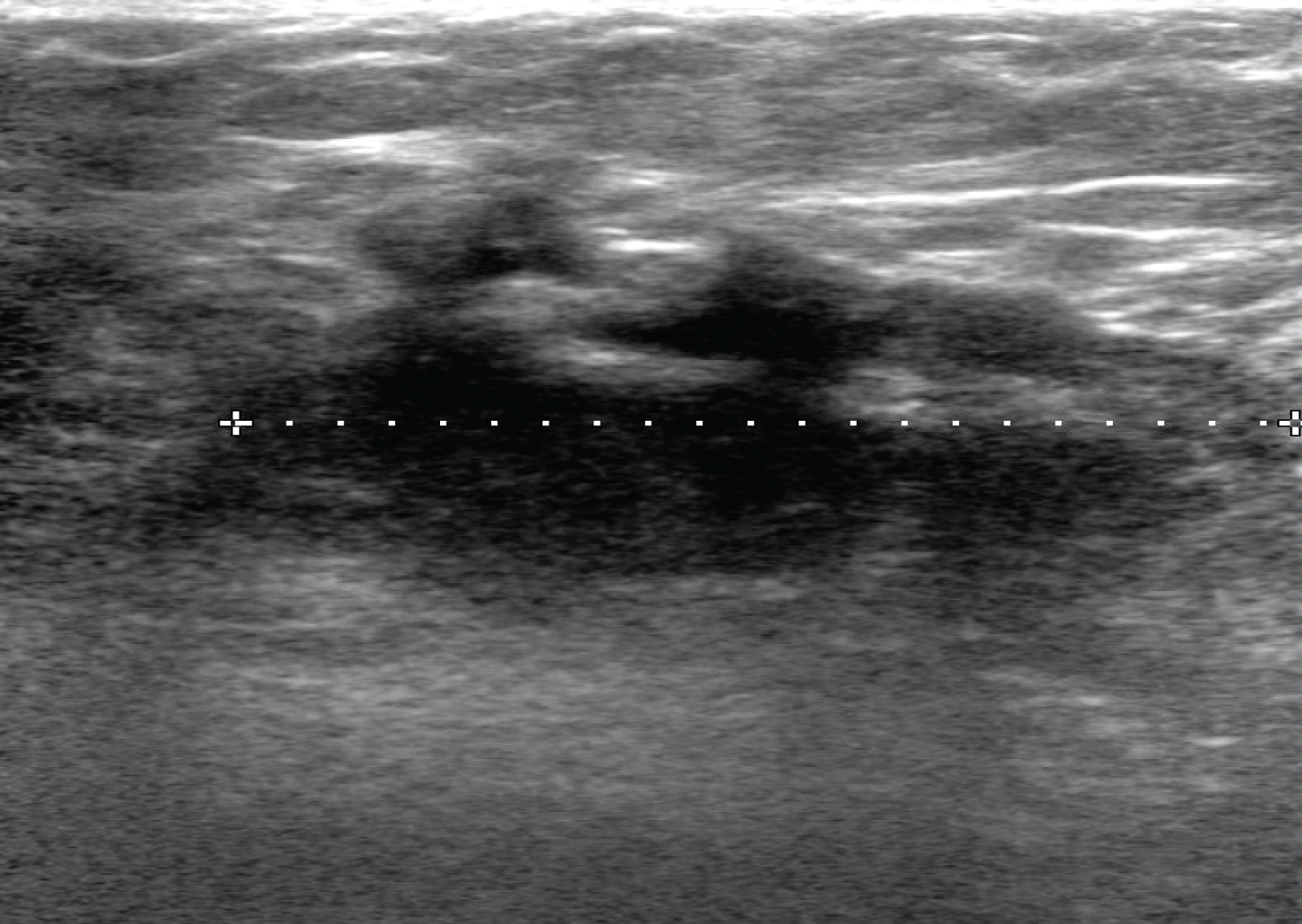 Figure 1: Ultrasound of the right breast showing an irregular hypoechoic mass with speculated margins seen at the axillary tail. There associated mixed posterior features.
View Figure 1
Figure 1: Ultrasound of the right breast showing an irregular hypoechoic mass with speculated margins seen at the axillary tail. There associated mixed posterior features.
View Figure 1
The patient subsequently underwent ultrasound guided wire localization followed by surgical lumpectomy of the lesion. The postoperative course was uncomplicated. The final histopathologic findings were suggestive. The final histopathologic findings were consistent with fibromatosis extending to the medial, anterior, lateral and posterior margin. The patient was followed up and at the time of this report, the disease had not recurred.
A 46-year-old woman presented with a palpable right breast mass and breast pain. There was no history of nipple discharge or skin changes. She had no family history of breast cancer but had a history of taking oral contraceptive pills for more than five years. She is known to have hypertension and diabetic mellitus on medications. There was no previous breast surgery or trauma to the breast or chest wall. Clinical breast examination revealed a well-defined mass at 5 o'clock on the right breast which was of 2 × 3 cm in size, firm in consistency, mobile and not fixed to skin. There were no skin changes. The axillary lymph nodes were not palpable.
The mass could not be included in the diagnostic bilateral mammogram. It was further assessed with ultrasound breast that showed an irregular hypoechoic mass with speculated borders seen in the subcutaneous tissue of the right inframammary region at 5 o'clock. It measured 3 × 1.2 cm (Figure 2). There was a thick right lymph node with a cortical thickness of 4.7 mm. The given BI-RDAS assessment was 5. Tru cut biopsy was done which showed a spindle cell lesion with dense fibrosis. Wide local excision was performed and histopathological examination showed an irregular spindle cell lesion formed of fairly uniform cells having plump vesicular nuclei with pale eosinophilic cytoplasm, infrequent mitotic figures and no atypia or necrosis. The stroma was variable from edematous to densely collagenous. A mild mononuclear infiltrate was present around some blood vessels. Red blood cell extravasation was seen. In some areas, there were thin walled dilated stag-horn like vessels. No epithelial component identified. No normal breast tissue seen. The lesion involved the anterior margin and was 7 mm from the deep margin. Other margins were clear. With immunohistochemistry, the spindle cells were positive for SMA, beta catenin and focally for desmin. They were negative for epithelial markers (CK-AE1/AE3 and CK-MNF-116), CD-34 and s-100. The final impression was of desmoid type fibromatosis. The patient was followed up clinically every 6 months, and at the time of this report, the disease had not recurred.
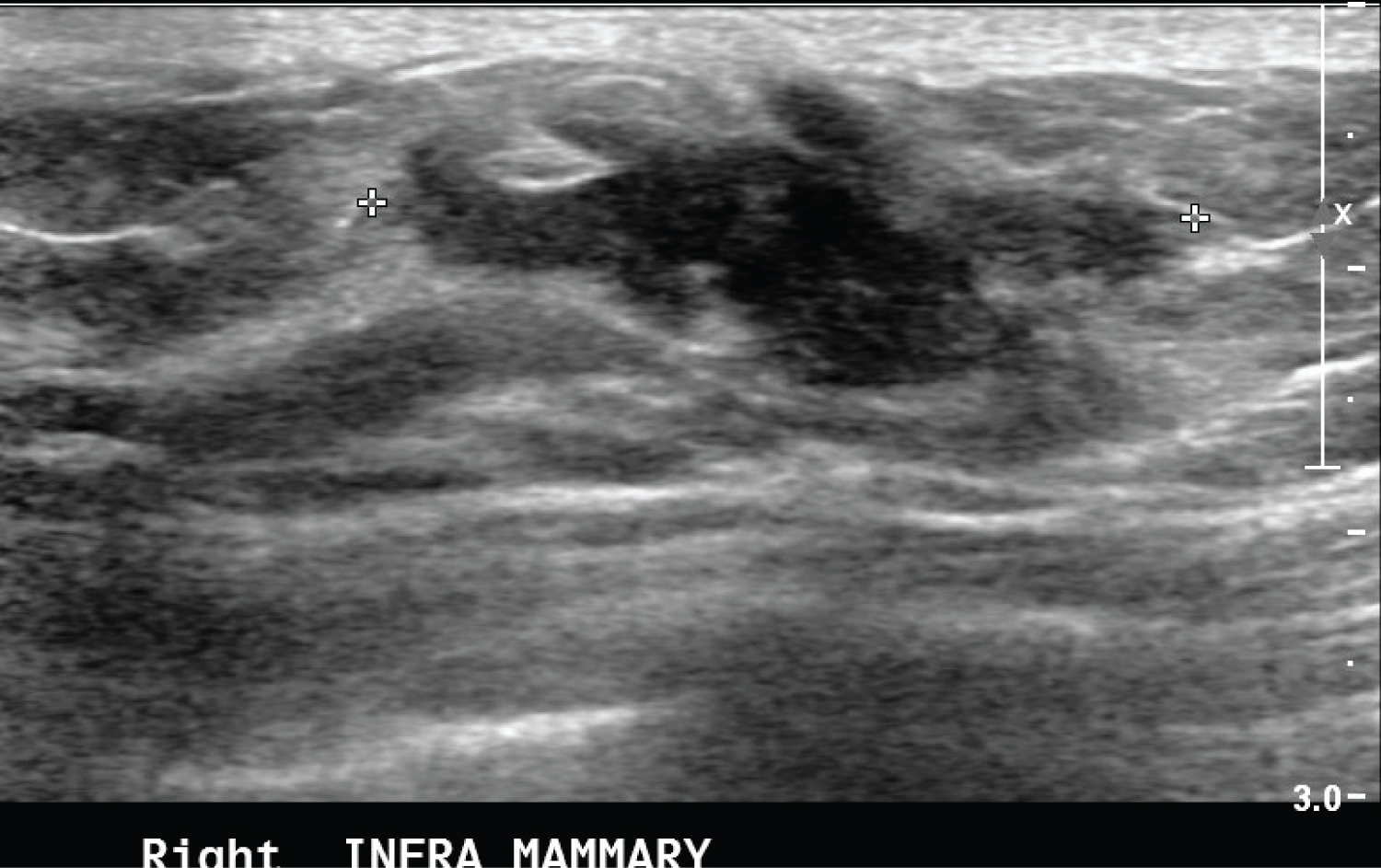 Figure 2: Ultrasound of the right breast showing an irregular hypoechoic mass with speculated margins seen at the right infra mammary fold. There associated mixed posterior features.
View Figure 2
Figure 2: Ultrasound of the right breast showing an irregular hypoechoic mass with speculated margins seen at the right infra mammary fold. There associated mixed posterior features.
View Figure 2
A 24-year-old woman presented with a palpable left breast mass that started 2 months before she presented to our breast surgical clinic. There was no history of nipple discharge or skin changes. She had family history of breast cancer (paternal cousin diagnosed with breast cancer at age of 27 year). She had no other illnesses and no history of oral contraceptive use. There was no previous breast surgery or trauma to the breast or chest wall.
Clinical breast examination revealed a well-defined mass at 2 o'clock on the left breast which was of 2 × 1 cm in size, firm in consistency, mobile and not fixed to skin. There were no skin changes and the axillary lymph nodes were not palpable.
The ultrasound assessment of left breast showed 2.3 × 0.4 × 2.2 cm mass at 2 o'clock (Figure 3). It was irregular with heterogeneous echotexture. Some of the borders were indistinct. It has peripheral vascularity. The axillary lymph nodes were sonographically normal. The given BI-RADS were 5. For which ultrasound guided biopsy was done. The pathology revealed spindle cell lesion suspicious for fibromatosis with additional presence of rare atypical epithelial cells (Figure 4). The patient subsequently underwent wide local excision of the lesion with uneventful postoperative course. The patient subsequently underwent wide local excision of the lesion with uneventful postoperative course. Final histopathology was reported as Fibromatosis. The patient has been on regular follow up now for six months with no apparent local recurrence.
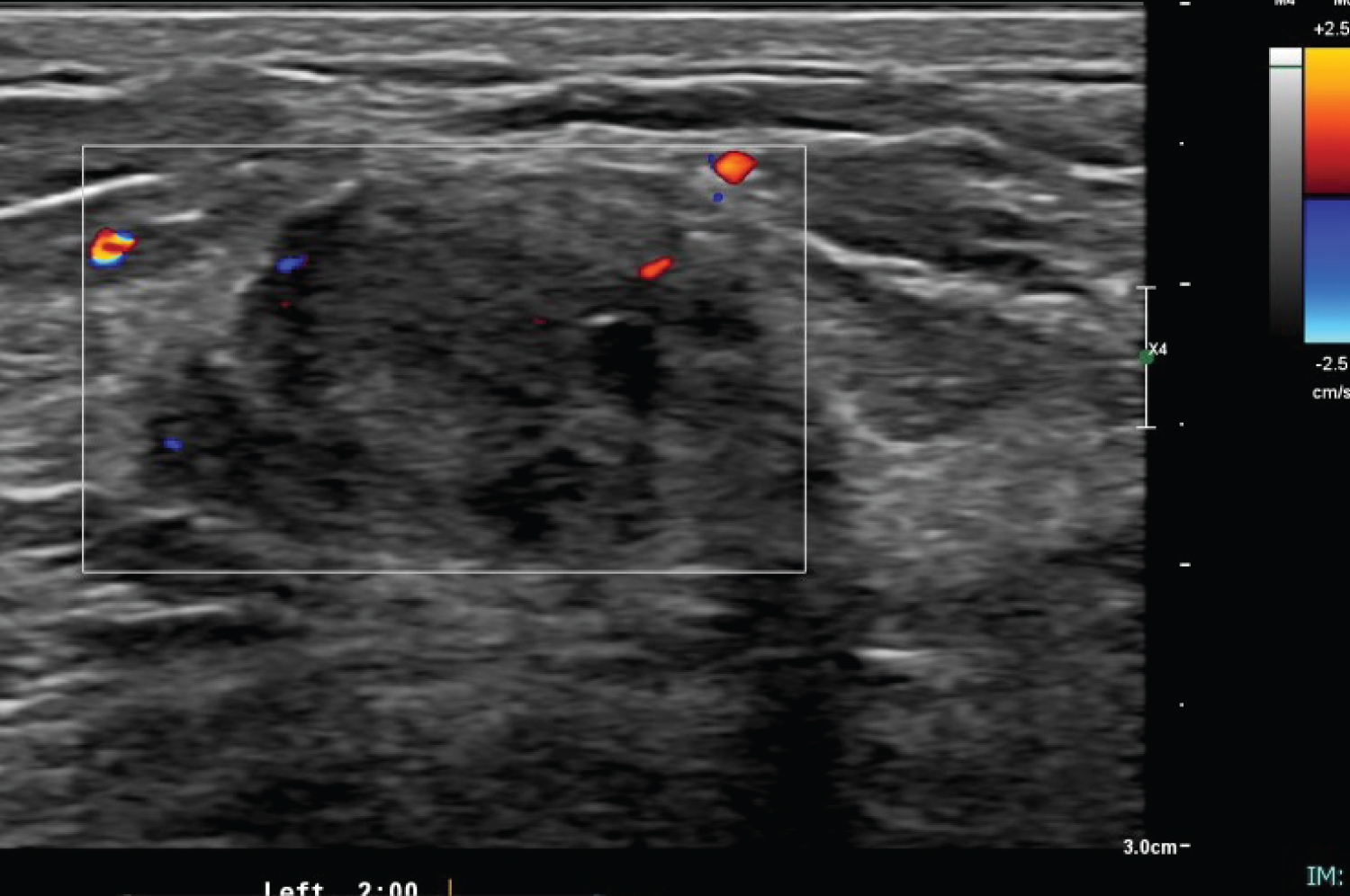 Figure 3: Ultrasound of left breast showing an irregular hypoechoic mass with indistinct margins seen at 2 o'clock. There associated mixed posterior features with peripheral vascularity. View Figure 3
Figure 3: Ultrasound of left breast showing an irregular hypoechoic mass with indistinct margins seen at 2 o'clock. There associated mixed posterior features with peripheral vascularity. View Figure 3
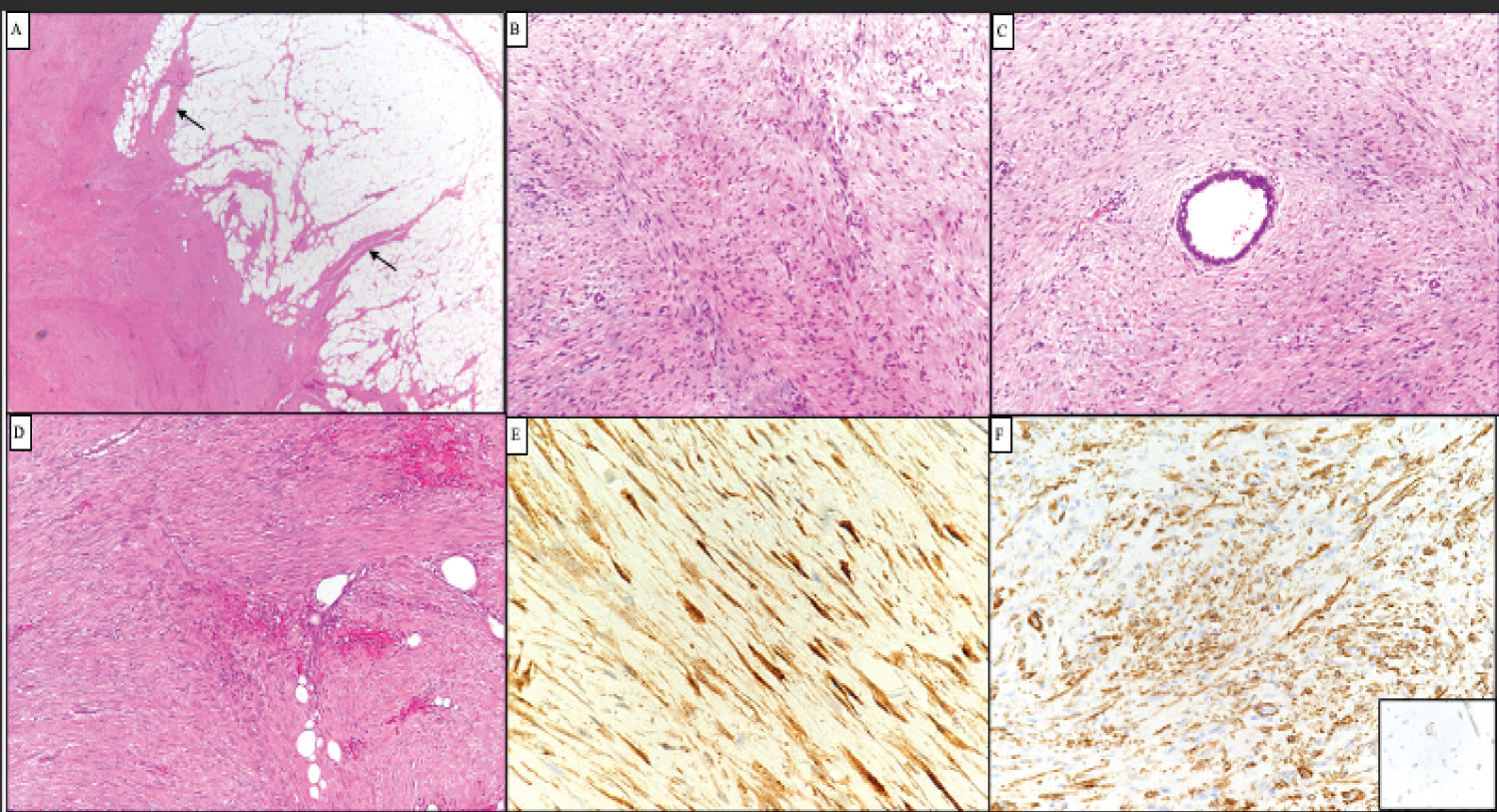 Figure 4: A) Representative photomicrograph of the tumors showing poorly circumscribed spindle cell neoplasm with finger like extensions into surrounding fat (A, arrows). The spindle cells are fairly uniform and exhibit fascicular arrangement; B) They surround epithelial structures and C) Fat; D) Tumor cells showed diffuse nuclear and cytoplasmic staining for beta catenin; E) They were also positive for smooth muscle actin; F) However desmin was negative (inset). View Figure 4
Figure 4: A) Representative photomicrograph of the tumors showing poorly circumscribed spindle cell neoplasm with finger like extensions into surrounding fat (A, arrows). The spindle cells are fairly uniform and exhibit fascicular arrangement; B) They surround epithelial structures and C) Fat; D) Tumor cells showed diffuse nuclear and cytoplasmic staining for beta catenin; E) They were also positive for smooth muscle actin; F) However desmin was negative (inset). View Figure 4
Fibromatosis or desmoid tumor is a rare disease, comprising of only 3% of soft tissue tumors and < 0.03% of all neoplasms [10,11]. It is clonal proliferation of fibroblasts and myofibroblasts, typically arising from the muscle, the fascia, and aponeurosis. It has aggressive behavior locally and high incidence of local recurrence [12]. These tumors do not metastasize. They have no capsule and tend to infiltrate into local structures. They have normal mitotic characteristics [3-5].
The most common extra-abdominal lesions are found in the shoulder girdle, pelvis, and thigh [7,11]. The breast is an unusual location for the development of this tumor. It represents 0.2% of all breast tumors and 4% of all extra-abdominal desmoid tumors. Bilaterality has been reported in 4% of cases [1,3,4]. All racial and ethnic groups are affected and no specific predilection is seen. Most of these cases were reported in young fertile females & rarely also occurs in men based on few study reports [13]. Mostly it occurs in the breasts of women aged between 13 and 80 years.
Fibromatosis of the breast may arise from the pectoralis muscle or fascia or the mammary tissue. Mammary fibromatosis appear to originate from fibroblasts and myofibroblasts within the breast parenchyma [8]. Although it does not metastasize, breast fibromatosis is frequently locally aggressive and is prone to recur (up to 35%), even after complete surgical excision with clear margins [1]. Multicentric and bilateral disease and recurrence at sites other than the primary have been reported [2,7,10,14]. Multicentricity in fibromatosis has been reported in 10% of cases [14]. Bilateral lesions are extremely rare, found in only 4% of patients [10]. Whether breast involvement is an extension from a primary site within the fibroaponeurotic fascia or the pectoral muscle or whether it results from fibroblasts originating from within the breast parenchyma is undetermined. However, fibromatosis arising within the breast parenchyma appears to represent a separate entity from extramammary fibromatosis, although both lesions may display a similar morphology. The extramammary lesions display a higher propensity for local recurrence compared to mammary fibromatosis [4,11].
Although the exact etiology of mammary fibromatosis remains unknown. Most of them occur sporadically but at least 30% of patients recount history of significant trauma to the involved area [4]. Antecedent trauma has been described at the site of fibromatosis in some patients and in association with saline-filled breast implants [4,15,16]. Few cases have been associated with Gardner's Syndrome, familial multicentric fibromatosis and familial adenomatous polyposis which suggests a genetic predisposition and probably alteration of the APC/beta-catenin pathway [1,4,15,17]. Some cases have been associated with sex steroid hormones, mainly estrogens (during childbearing age, the disease tends to be more "cellular," more mitotically active, with a larger amount of mild cellular atypia), suggesting a hormonal correlation [7]. It is important to recognize mammary fibromatosis as it presents a big dilemma for the clinician, because it mimics cancer clinically, radiologically and sometimes cytologically [4].
Clinically, breast fibromatosis almost always presents with a painless, solitary, firm or hard tumor that suggests carcinoma on clinical examination as the case with our patients. Rarely, the tumor is non-palpable and detected initially by mammography [4]. Skin dimpling and retraction are relatively common signs of breast fibromatosis & is caused by fibrous tissue contraction vs. desmoplastic reaction, which is similar to tethering associated with malignancy [2]. The tumor size may range from a few millimeters to 10 cm (the average size being 2.5 cm). Small-sized tumors may be asymptomatic and show no signs and symptoms. Neither nipple discharge nor axillary lymphadenopathies commonly occur with it [8,9]. In our three cases, there was no skin involvement.
On mammography, breast fibromatosis often appears as an irregularly shaped, noncalcified, high-density mass with spiculatedmargins [18]. Radiologic evaluation of our second and the third cases were classified as BIRADS 5 and the first case as BIRADS 4C. On ultrasound, breast fibromatosis frequently appears as a poorly defined, hypoechoic mass with a thick echogenic rim and a posterior attenuation. The clinical presentation and the radiological appearance of breast fibromatosis are highly suspicious for breast carcinoma. There are 2 cases reported in the literature: Both patients underwent radical mastectomy because of an erroneous clinical diagnosis of breast carcinoma [19]. Pre-operative diagnosis of fibromatosis by FNAC is rare. Nevertheless, fine needle aspiration cytology, although not entirely specific, may be a source of important information in patients with breast fibromatosis. In particular, it confidently allows the exclusion of breast cancer and other more common diseases and is useful in planning a surgical approach to the lesion [16]. McKinnon, et al. [12] noted that the correct diagnosis of desmoid tumors was made preoperatively in only 50% of cases and that, even after biopsy, the diagnosis was often confused with low-grade fibrosarcoma, confirming the difficulty of diagnosis for this pathology*.
Since, fibromatosis is an infiltrative proliferation of fibroblastic and myofibroblastic cells, the positivity for vimentin and smooth muscle actin was not surprising. Curiously, fibromatosis in the breast differs from fibromatosis arising in other parts of the body due to its hormone receptor profile. Although 30% of extramammary fibromatosis are positive for estrogen receptors, only one of the previously reported cases of mammary fibromatosis expressed hormonal receptors [20]. Because of the consistent absence of immunoreactivity for estrogen and progesterone receptors in mammary fibromatosis, a positive reaction for these receptors in spindle cell neoplasms of the breast might be helpful in excluding fibromatosis from its differential diagnoses [20]. Another major concern about mammary fibromatosis is the exclusion of the diagnosis of metaplastic carcinoma, because spindle cell tumors with a myoepithelial immunophenotype may be diagnosed as metaplastic carcinoma even with weak or absent cytokeratin expression. In the present report, both epithelial (AE1/AE3) and myoepithelial markers (CD14 and p63) were negative [15]. Mammary fibromatosis presents macroscopically as a dense, poorly vascularized, hard, rubbery, grayish-white mass. The cut-surface usually shows an nonencapsulated mass with fibrous, gray and white nodular parenchyma [21]. Microscopically, fibromatosis is composed of uniform, spindle shaped fibroblasts forming sweeping or interlacing fascicles entrapping ducts and lobules with an infiltrative edge. The degree of cellularity varies, ranging from relatively cellular to predominantly collagenized lesions. Devouassoux-Shisheboran, et al. studied the morphofunctional features of 33 cases of fibromatosis and showed that the cellularity of the lesion varied with the patient's age. Lesions in the younger patients (childbearing age) were significantly more cellular than those of the perimenopausal and postmenopausal groups, it displayed larger proportion of cells with mild atypia and were mitotically more active. Compared to lesions in the childbearing group, 15 lesions in the perimenopausal and postmenopausal patients were significantly more fibrous and presented with prominent inflammatory cells. Immunohistochemically, fibromatosis exhibits positivity for smooth muscle actin and vimentin and negativity for cytokeratin, estrogen, progesterone and androgen receptors (ER, PR and AR) [22]. Numerous reports showed that even though extramammary fibromatosis usually exhibit positivity for ER and PR, mammary fibromatosis is consistently devoid of such receptors [4,22], desmin is rarely positive, whereas S100 and CD34 are usually negative. Immunohistochemical staining usually shows positivity for B-catenin in 70-80% of cases few studies have shown that many sporadic fibromatosis cases have CTNNB1 mutation.
Management remains controversial because of the limited data due to its low incidence. Wide local excision with clear margins is considered the standard of care. Because of the stellate configurations and grossly inapparent extensions of most lesions. None of the literature we reviewed reported any mammary fibromatosis metastasis or death of patients from their disease [1,4]. However, malignant transformation of fibromatosis following radiation therapy was reported [17]. As breast fibromatosis do not demonstrate metastatic capabilities, axillary dissection is not performed [17]. The positive margin seems to be associated with recurrent disease [3], but not all positive margins were recurrent [4]. Positive excision margins and intralesional excisions are associated with a greater rate of recurrence. Younger age and larger tumor size are also associated with an increased risk of recurrence [23]. A literature review conducted by Trey Thomas, et al. suggests that patients should undergo quarterly clinical examination for a minimum of three years; as available literature suggests the majority of local recurrences manifest within this time frame [17]. In this time frame, close follow-up is important [7]. Because fibromatosis is not cancer, it has a 100% survival rate [24].