Objective: To study the characteristics of surgical site infections (SSI) in digestive surgery in Brazzaville.
Patients and Methods: This was an analytical, cross-sectional, and multicenter study conducted from March 1 to September 30, 2024, in the digestive surgery departments of the University Hospital Center (CHU) and general surgery departments of five referral hospitals in Brazzaville. All hospitalized patients aged at least 18 years who underwent abdominal surgery were included in the study. Patients were followed up within 30 days of surgery.
Results: During the study period, 493 patients were included, 73 cases of SSI were recorded, representing a frequency of 14.8%. Was the center with the most cases of SSI were found in the university hospital (58 patients or 79.4%). The median age was 41 years. Male sex was predominant with a sex-ratio of 1.35. Among the SSIs, 74% were superficial; 20.5% deep and 5.5% organ. Acute generalized peritonitis was the most common pathology of infected patients (60.3%). Of the 73 samples analyzed, 70 cultures were positive, representing 95.8%. Escherichia coli were the most identified microorganism (36.5%), followed by non-coagulase Staphylococcus (15%) and Klebsiella pneumoniae (12.2%). Multivariate analysis showed that SSI was statistically associated with ASA score, operative mode, and NNISS score.
Conclusion: SSIs represent a major public health problem. Prevention relies on rigorous epidemiological surveillance and compliance with good perioperative hygiene practices.
Infections, Surgical site, Digestive surgery, Brazzaville
Surgical site infections (SSIs) are a major challenge in surgical interventions and care, in terms of increased risk of mortality in patients and threats to the quality of health systems [1, 2]. They represent one of the main complications of surgery [3]. They are considered the leading nosocomial infection in surgical patients and the third most common in all hospitalized patients, after urinary tract infections and respiratory infections [4]. The infection is considered nosocomial when it appears during or following hospitalization in a patient, as long as it was not present at admission and occurs within at least 48 hours after admission or when it occurs longer than the incubation period when it is known [1, 5].
SSIs occur within thirty (30) days of surgery or within one year if there was an implant or prosthesis placed that affects the skin, subcutaneous tissues, organs, or spaces manipulated during surgery [1]. They have a significant impact on the length of hospital stay, increased healthcare costs, and mortality rates [6- 8]. The impact of SSIs extends beyond the patient, casting a shadow on the broader healthcare landscape by straining human resources and compromising patient safety [9]. With an overall mortality rate of 3%, the occurrence of SSIs is influenced by a set of predisposing factors, including: factors specific to the patient (age, general health and immunosuppression) and factors related to the surgical procedure (colonization of the incision site, duration and type). Regarding this second category of factors, the literature mentions that urgent surgical procedures, prolonged surgical times, the use of non-absorbable sutures, the presence of foreign bodies, excessive subcutaneous electrocautery, significant blood loss and hypothermia increase risk of SSI [1]. SSI is also linked to the hospital context, in particular, the level of hygiene and the operating conditions [10, 11]. Diagnosis is generally easy when it is a superficial infection (parietal suppuration) but difficult when the infection is deep or involves the spaces manipulated during the procedure [12]. Microbiologically, SSI can be monomicrobial or polymicrobial [13]. Bacteria are the causative microbial agents in the majority of cases. They are inoculated during surgery and originate from non-sterile skin or mucous membranes affected during surgery. In addition, microbial agents can originate from several routes of contamination and transmission such as air, contact and droplets, from vectors such as surgical staff, the operating room environment and all instruments that come into contact with the surgical site [14]. Their management is currently made difficult by the presence of germs that are increasingly resistant to common antibiotics [15].
SSIs are thus responsible for a considerable increase in hospital morbidity, length of hospital stay, mortality and therefore generate additional costs [16], constituting a problem of primary importance in terms of surgical care.
SSIs represent 1.7% of surgical procedures in Europe [17]; between 0.5 and 3% in the USA [18] and 3.18% in China [19]. In the Middle East, particularly in Saudi Arabia, SSIs are reported in orthopedic procedures and traumatic laparotomies at respective frequencies of 2.5% and 12.9% [20]. In Africa, they constitute a real public health problem [21]. Its prevalence is generally estimated at 6.8 to 26% [22- 25]. However, it varies between 6 and 40% in sub-Saharan Africa in particular [22- 25]. In Congo, a preliminary single-center study on SSIs, carried out in the digestive surgery department at the Brazzaville University Hospital, showed a frequency of 3.1% from 2021 to 2023 [26]. In order to improve the reliability and generalizability of the results of this study, we proposed to broaden the scope of action within the framework of an analytical and multicenter study with the aim of developing evidence-based strategies to mitigate the impact of SSI, in order to improve their management.
The specific objectives were to determine the frequency of SSI in digestive surgery, describe the sociodemographic and clinical characteristics of SSI in patients with SSI, list the main germs responsible for SSI and identify the factors associated with the occurrence of SSI.
Cross-sectional, multicenter, prospective data collection analytical study.
The study was carried out from March 1 toSeptember 30, 2024, in Brazzaville, capital of the Republic of Congo, in the surgical departments of five hospitals: the digestive surgery department of the Brazzaville University Hospital and four general surgery departments of these referral hospitals.
The choice of these hospitals was based on a reasoned method, due to the attendance of these centers by the population and the existence of surgical services in these centers.
All patients operated on for digestive pathology in the surgical departments of the above-mentioned hospitals, aged at least 18 years regardless of gender and having consented to the study were included in the study.
Patients who have undergone proctological surgery.
Patient sampling technique was exhaustive and consecutive sampling during the study period.
In the general and digestive surgery departments of the different centers involved in the study, all patients included were divided into two groups: group 1: patients operated on for a digestive condition during the study period and experiencing an SSI; group 2, patients operated on for a digestive pathology during the study period, who did not experience an SSI.
Patients not meeting the inclusion criteria and those lost to follow-up during follow-up.
Patients hospitalized in the surgical department each have a medical record in which all administrative, clinical, paraclinical, diagnostic and therapeutic data are recorded, as well as data from the examination during the check-up. Electronic forms have reduced bias in data entry and facilitated data collection. Information concerning the intraoperative period was taken from the surgical report register and anesthesia sheets.
The dependent variable of the study was SSI. The independent variables included sociodemographic (age, sex, occupation, socioeconomic level), anamnestic, clinical and biological data.
The diagnosis of SSI was mentioned by general practitioners and confirmed by surgeons who performed the surgical procedures in the 5 hospitals.. The diagnosis of SSI was based on a sample for bacteriological examination with antibiogram. The criteria for judging SSI were those of the CDC ATLANTA of 1992/1999 describing the criteria for the diagnosis of SSI [3]. Patients were seen and examined in hospital and reviewed after discharge, in outpatient consultations on the 15th and 30th day following surgery.
Preoperative data collected were: administrative data, clinical and laboratory information, preoperative diagnosis and treatment, ASA score and NNISS score.
Intraoperatively, these were: type of surgery, duration of the procedure, surgical technique used, and treatment received by the patient and the number of people in the operating room. Postoperative data consisted of clinical data on postoperative infection, treatment, length of hospital stay, other complications and patient outcome. Regarding biological data, they were based on cytobacteriological examination of pus and antibiogram of positive results. Pus samples were taken between 8 a.m. and 12 p.m.
The study adhered to rigorous standards throughout. Ethical approval was obtained from the Health Sciences Research Ethics Committee of the General Delegation for Scientific and Technological Research of the Republic of Congo.
Data entry was performed using Epi-data software, version 3.0. The database was cleaned on an Excel page (xls) and analyzed using PASW Statistic 18.0 software.
Categorical variables are presented as frequencies and percentages. Statistical analyses, including the Sokal and Rohlf S test and the Chi-square test, were used to determine the association between qualitative variables. To establish the association between SSIs and environmental, clinical, and personal factors, simple and adjusted odds ratios (OR) (logistic regression) were calculated.
Factors associated with SSI were considered as: risk factors when OR>1 and the 95% confidence interval did not include 1; protective factors when OR < 1 and the 95% confidence interval did not include 1; there was no association when OR = 1. Furthermore, a multivariate logistic regression was also considered when at least three (03) factors were statistically associated with SSI in the context of a crossover with two variables using the Chi2 test. Logistic regression made it possible to eliminate confounding factors and establish a parsimonious model where all explanatory variables (x) explain the model (y). The test was statistically significant when the p-value was strictly less than 0.05 (p < 0.05).
During our study, we found73 cases of SSI in 493 surgical procedures, representing a frequency of 14.8%. These were 42 men (57.5%) and 31 women (42.5%) (sex ratio, 1.35).
The distribution of SSIs by center is shown in Figure 1.
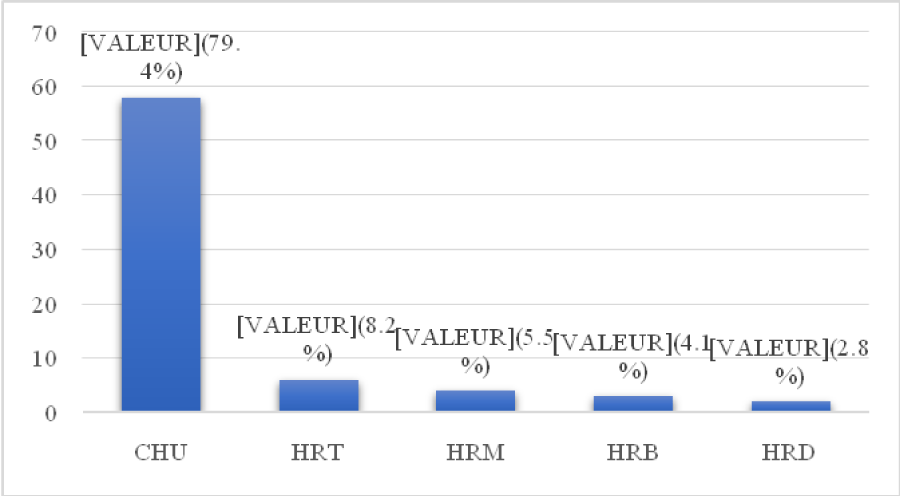 Figure 1: Distribution of SSIs by center. CHU: University Hospital Center; TRH: Talangaï Reference Hospital; MRH: Makélékélé Reference Hospital; BRH: Bacongo Reference Hospital; DRH: Djiri Reference Hospital
View Figure 1
Figure 1: Distribution of SSIs by center. CHU: University Hospital Center; TRH: Talangaï Reference Hospital; MRH: Makélékélé Reference Hospital; BRH: Bacongo Reference Hospital; DRH: Djiri Reference Hospital
View Figure 1
Their median age was 41 years (Q1 = 29 years; Q3 = 55 years) (range: 18-78 years).
Among the 73 patients with SSI, 35 (48%) were from the informal sector, 21 (28.7%) were from the formal sector and 17 (23.3%) were unemployed. Regarding the economic level, 40 (55%) of them had a medium economic level, 22 (30%) a high economic level and 11 (15%) a low economic level.
The preoperative clinical picture of infected patients was generalized acute peritonitis in 60.3% of cases (Table 1).
Table 1: Distribution of patients with SSI according to the preoperative clinical picture. View Table 1
Among the 73 patients presenting with SSI, we found60 cases of emergency surgical interventions, i.e. 82.2%, and 13 cases of scheduled surgical interventions (scheduled surgery) (17.8%).
As for the duration of preoperative hospitalization, in 75% of cases patients were operated on the same day, 15% between 2-7 days and 10% for durations greater than or equal to 8 days.
Regarding the preoperative bath, 61 (83.6%) did not take one compared to 12 (16.4%) who did.
The duration of surgical intervention in infected patients was more than one hour in 66 cases (90.4%), and at most one hour in 7 cases (9.6%). As for drainage, sixty-eight infected patients (93.2%) had a drain, while 5 patients (6.8%) had not had one.
Figure 2 shows the distribution of infected patients according to ASA score.
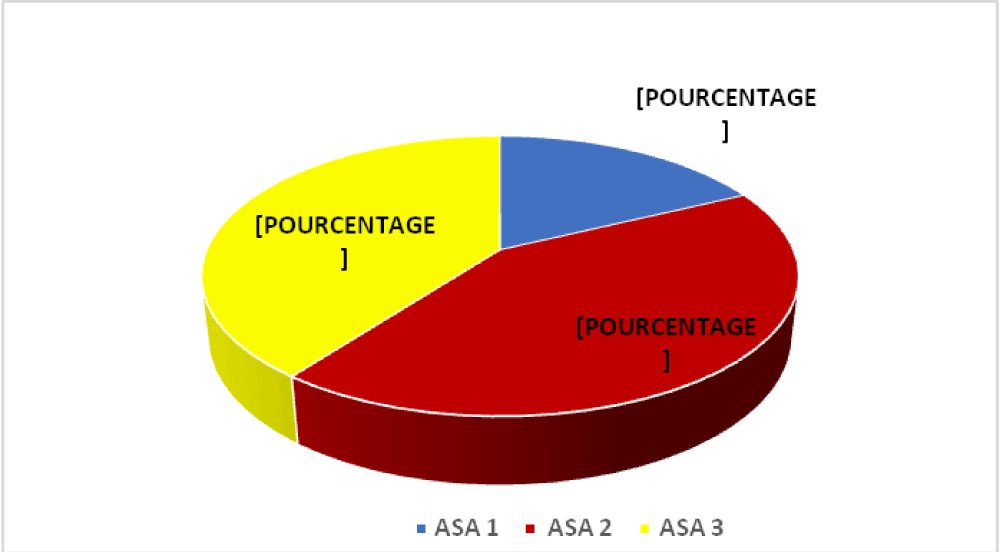 Figure 2: Distribution according to ASA score.
View Figure 2
Figure 2: Distribution according to ASA score.
View Figure 2
Regarding the type of digestive surgery, it was considered dirty in 71.2% of infected patients. Regarding the NNISS score, among the 73 infected patients, 10 of them (13.7%) presented an NNISS score of 3 (Figure 3).
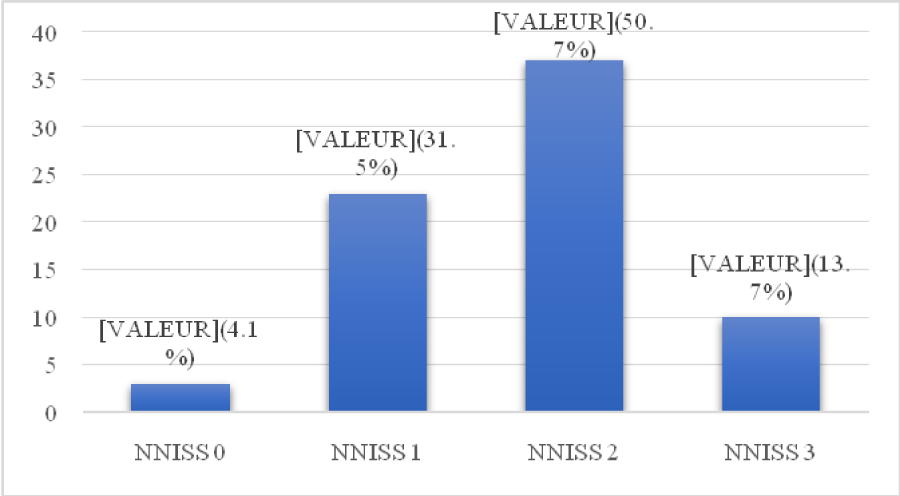 Figure 3: Distribution according to NNISS score.
View Figure 3
Figure 3: Distribution according to NNISS score.
View Figure 3
The median time to onset of SSI was 4 days (Q1 = 4 days; Q3 = 5 days) (range: 3-18 days). Regarding the location of SSI onset, 67 patients (91.8%) experienced SSI during hospitalization, 6 (8.2%) after hospital discharge. Table 2 highlights the mode of diagnosis of SSI.
Table 2: Distribution according to the method of diagnosis of SSIs. View Table 2
SSIs were superficial in 74% of infected patients (Figure 4a), deep in 20.5% (Figure 4b) and organ-related in 5.5%.
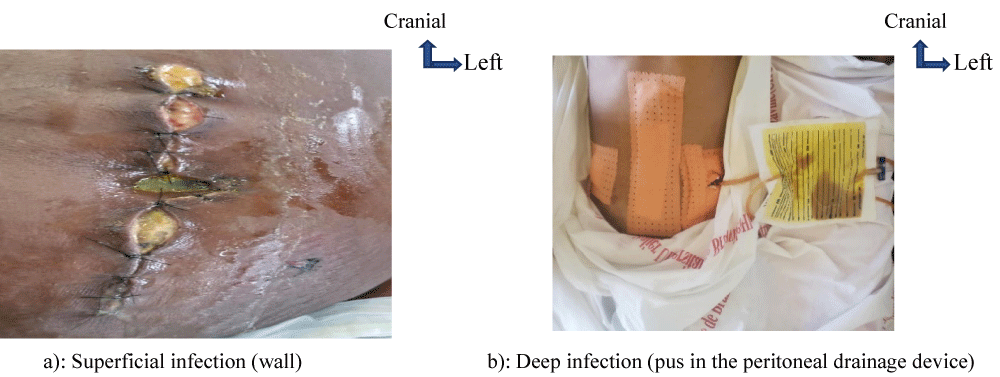 Figure 4: Type of infection. a): Superficial infection (wall) b): Deep infection (pus in the peritoneal drainage device)
View Figure 4
Figure 4: Type of infection. a): Superficial infection (wall) b): Deep infection (pus in the peritoneal drainage device)
View Figure 4
Bacteriological analysis, which resulted in 70 positive cultures and 3 sterile cultures, identified 74 germs (Table 3). Polymicrobial associations included: Escherichia coli + Staphylococcus aureus; Klebsiella pneumoniae + non-coagulase Staphylococcus; Proteus vulgaris + non-coagulase Staphylococcus.
Table 3: Germ distribution. View Table 3
The antibiogram identified eleven SSIlated germs (Table 4). In addition, we identified a non-albicans candida fungus. It was sensitive to econazole and miconazole and resistant to itraconazole.
Table 4: Antibiogram. ; The first number = number of strains sensitive to the antibiotic in question.; The number in parentheses = number of resistant strains (resistant strains + strains of intermediate sensitivity). View Table 4
All patients with an SSI received local care (daily or twice-daily dressing) and probabilistic antibiotic therapy, secondarily adapted to the results of the antibiogram. Six patients underwent further surgery.
The median length of hospital stay was 12 days (Q 1 = 7 days; Q 3 = 21 days) for patients with SSIs, compared to 5 days (Q 1 = 3 days; Q 3 = 7 days) for those without SSIs. We found 7 deaths among the 73 patients with SSIs (9.5%).
Multivariate analysis after logistic regression determined the following predictive factors for SSI: ASA score, operating mode and NNISS score (Table 5).
Table 5: Predictive factors associated with SSI. View Table 5
Most SSI patients had ASA 2 (42.5%) and ASA 3 (39.7%) scores. Emergency surgery patients (82.2%; p = 0.019) developed more SSIs. In addition, we found an increased risk of SSI with increasing NNIS score (NNISS 1: p = 0.049; NNISS 2: p = 0.049; NNISS 3: p = 0.049).
Our results mainly showed that out of 493 abdominal surgical procedures performed in the surgical departments of 6 hospitals in Brazzaville, 73 patients developed an SSI. The study highlighted the link between SSI and sociodemographic characteristics, clinical, bacteriological, therapeutic and evolutionary aspects.
The frequency of SSI in our study was 14.8%. It is close to those found in Mali [25], Togo [21] and Senegal [27] which are respectively 10.9%; 11% and 13.6%. However, this rate is much lower in developed countries. It is around 1.7% in Europe [17], 3.18% in China [19] and varies between 0.5 and 3% in the USA [18]. Since SSI is multifactorial, this difference can be explained by less invasive therapeutic innovations in developed countries with the laparoscopic approach reducing their SSI rate and the mandatory preparation of the patient (bath). On the other hand, in Africa due to the weakness of the technical platform, laparotomy is frequently performed with a longer hospital stay. We also note the promiscuity in the hospitalization rooms, the lack of dressing materials, the insufficiency of hospital linen (sheets, clothes), which means that each patient uses what they have. Most often, a sheet is used for several days without being changed. Nurses sometimes move from a septic wound to a clean wound during dressings. Insufficient preparation of emergency operated patients is found, as well as the lack of rigor in asepsis and antisepsis measures and the precarious state of the technical installations in the operating room.
CHU was the center most affected by SSIs (79.4%). This could be explained by the fact that it represents the largest hospital center in the city and performs the greatest number of interventions. Due to budget cuts and the significant decrease in funds allocated to the optimal functioning of operating theaters in recent years, rigorous and periodic surveillance of nosocomial infections must be an absolute priority.
The median age is 41 years. Our results are similar to those found by other African authors [25, 28, 29]. On the other hand, Pivot, et al. [30] in France estimate that SSI occurs frequently at advanced ages with an average age of 54 years. This difference is explained by the aging of the European population, aging leading to a decline in immunity.We found a male predominance in our series. Bengaly, et al. [31] report the same result as us, with a sex ratio of 1.66.
Acute generalized peritonitis represents the first digestive pathology responsible for SSI in our work. These results are similar to those described in the literature [25, 26, 29]. This can be explained by the fact that it is a dirty surgery with a significant risk of contamination of the wall. In addition, when the lavage is not effective, there is a risk of formation of a residual collection. Acute generalized peritonitis is followed in our study of tumor and occlusive pathologies. Tumors and occlusions are the pathologies for which a digestive stoma was performed intraoperatively in the majority of cases. Poor management of the stoma (defect and/or non-device due to lack of financial means) by patients and their entourage could explain the occurrence of SSI.
The mean time to onset of SSI of 5 days in our study is similar to those reported in the African literature [26, 28, 31], varying between 5 and 10 days. This mean time to onset is explained by the incubation time and the inflammatory process but also the high percentage of interventions that are part of dirty and contaminated surgery.
The superficial site was the most frequent location of SSIs during our study, 74%. This rate is close to those reported by the ECDC [17] and Note, et al. [26]. In contrast, Mahamadou andal [37] in Niger on a work carried out in three departments (digestive surgery, urology and orthopedics) found a higher rate for the deep seat. This difference can be explained by the multidisciplinarity of his study.
Escherichia coli was the dominant germ as revealed in several works [8, 29, 30]. It is a commensal germ of the digestive tract that can infect the abdominal wall through the stoma and by direct contamination of the peritoneal cavity following perforation of the digestive tract.Regarding the antibiogram, it was found to be sensitive to Meropenem and Imipenem, which is in accordance with the study of Tririsha, et al. [33] in India.
We have observed resistance of germs to the majority of antibiotics. This can be explained by the misuse of antibiotics as well as self-medication. Indeed, so-called blanket antibiotic therapy does not reduce SSIs but, on the contrary, promotes bacterial resistance to antibiotics by selecting resistant mutants.
The median length of hospitalization was 12 days for infected patients. This duration is relatively short compared to those of Diarra andal [25] in Mali, Note, et al. [26] in Congo who obtained 21 and 19 days respectively. This difference could be explained by prolonged parenteral antibiotic therapy and close local care (twice daily) in case of SSI. The overall case fatality rate in our series was 9.5%. Note, et al. [26] in Congo reported a case fatality rate of 12.5%. These results are close to ours.
Multivariate analysis allowed us to establish a predictive model for SSI. The predictive factors retained after logistic regression were: ASA score, operating mode and drain placement.
We found an increase in the SSI rate with the ASA score (p = 0.0000). Bengaly, et al. [31] in Mali, Karima, et al. [32] in Morocco, Rattatasoa, et al. [38] in Madagascar, Astagneau, et al. [35] in France, also consider the ASA score as a factor influencing the occurrence of SSI. The severity of pre-existing pathologies would explain this. Furthermore, patients operated on in emergency developed SSI the most. Mahamadou, et al. [37] in Niger as well as Note, et al. [26] in Congo made the same observation. Similarly, emergency interventions influence the occurrence of SSI in our study as in those of Diarra, et al. [25] in Mali and Flouchi [35] in Morocco. The lack of preparation (bathing) of patients in the emergency room could explain this.
The NNISS score assesses the risk of acquiring a post-operative infection.The SSI rate stratified on the NNISS index was 4.1% (NNISS = 0); 31.5% (NNISS = 1); 50.7% (NNISS = 2) and 13.7% (NNISS = 3). During our study we recorded twelve (12) patients with a NNISS score of 3, ten of whom presented an SSI.We found an increased risk of SSI with NNISS score. This result corroborates with the data of Escutnair, et al. [36].
The NNISS score is multifactorial. It is obtained by combining the following three risk factors: duration of the procedure, the patient’s ASA score and the ALTMEIER contamination class. The parameters constituting this score could explain the increased risk of SSI occurrence. Indeed, the extension of the duration of the procedure exposes patients to the risk of contamination of the surgical site. In addition, the existence and severity of underlying pathologies would increase the risk of SSI occurrence. Finally, the dirtier the surgery, the higher the risk of SSI occurrence [9, 34] due to contamination of the wall and cavities during the procedure.
Our study of SSIs in digestive surgery in Brazzaville was conducted in the general surgery departments of four referral hospitals and the digestive surgery department of the Brazzaville University Hospital. These five departments are in fact the departments responsible for digestive surgical pathologies. Note, et al. [26] conducted a single-center study on SSIs at CHU. We believe that the multicenter nature of this study provides a comprehensive view of SSIs in digestive surgery in Brazzaville. The prospective data collection of this study ensures good quality of our results. The collection of information is contemporary with the events described. The exhaustive sampling allowed us to include 493 patients. Mahamadou, et al. [37], Kanassoua, et al. [21, 38] report samples of 485 and 271 patients respectively. Electronic forms reduced bias in data entry and facilitated data collection. However, the period of our study constitutes a bias in the diagnosis of SSIs in patients wearing prostheses. Indeed, SSIs can occur up to one year after the placement of prosthesis.
SSIs are common in digestive surgery in Brazzaville. The median age is 41 years, with a male predominance. These are mostly early and superficial SSIs. Escherichia coli, non-coagulase Staphylococcus, and Klebsiella pneumoniae are the most SSIlated bacteria. SSIs were influenced by the ASA score, the surgical procedure (emergency), and the NNISS score. Despite appropriate antibiotic therapy, local care, and surgical reintervention in severe cases, SSIs still carry significant morbidity and mortality. Prevention relies on rigorous epidemiological surveillance and adherence to good perioperative hygiene practices.
None.
The authors confirm their contribution to the article as follows: study design and development: Massamba Miabaou Didace; data collection: Boudzoumou Michael A. Sabine, Elion Ossibi Pierlesky, Nzaka Moukala Carmiche, Tsouassa Wa Ngono Giresse, TatyTaty Raphael; analysis and interpretation of results: Massamba Miabaou Didace, Note Madzélé Murielle Etiennette Julie; preparation of the draft manuscript: Massamba Miabaou Didace, Note Madzélé Murielle Etiennette Julie, Masamba Alphonse. All authors reviewed the results and approved the final version of the manuscript.