Background: Enterocutaneous fistula (ECF) poses a significant challenge to surgeons following abdominal surgeries. This study aimed to evaluate the factors associated with management outcomes of ECF patients.
Methods: This was a longitudinal analytical study conducted at 3 tertiary hospitals, from August 2022 to July 2023. A non-probability sampling technique was used, and data analysis was performed using SPSS version 26.
Results: The study included 60 patients with a mean age of 40.3 ± 14.5 years, with over one-third of participants aged 45 years and above. Of all patients, 82% experienced unfavorable outcomes, with 30% succumbing to death, while 73.3% of survivors experienced an extended hospital stay due to ECF-related complications. Multivariate logistic regression analysis identified small bowel ECF (AOR: 18, 95% CI: 1.311-260.3), anemia with a hemoglobin level below 11 g/dL (AOR: 6, 95% CI: 1.115-32.42), and age above 45-years-old (AOR: 24, 95% CI: 1.268-457.5) as independent predictors of unfavorable outcomes in patients.
Conclusion: Enterocutaneous fistula is not an uncommon complication among patients who have undergone abdominal surgery. Small bowel enterocutaneous fistulas, age above 45 years, and anemia with a hemoglobin level below 11 g/dl are significant factors associated with unfavorable outcomes among patients with ECF. These findings emphasize the complex nature of managing ECF and the crucial role of addressing both the primary condition and its associated complications to enhance patient outcomes.
Enterocutaneous fistula, Management outcomes, Unfavorable outcomes, University of Dodoma
Dealing with enterocutaneous fistula (ECF) is a complex challenge that poses significant consequences in surgical wards [1]. Managing this condition effectively requires navigating through intricate clinical scenarios. The first step in this process is stabilizing the patient's condition. It's important to recognize that electrolyte imbalances, infections, and malnutrition can pose serious risks to patients. Therefore, identifying and managing these factors is critical to saving lives. Maintaining a balance in electrolyte and fluid levels is crucial and requires constant monitoring due to the rapid onset of imbalances. The location of the fistula and the amount of output determine the extent of electrolyte loss. As research has highlighted [2,3], swift correction of any identified derangements is imperative.
In cases involving septic patients, the identification and accurate treatment of the source of infection is paramount. Sepsis is estimated to contribute to approximately two-thirds of ECF-related fatalities, with intra-abdominal abscesses being a prevalent source of septic complications. A significant proportion of patients will necessitate parenteral nutrition to address their nutritional needs. However, if the fistula is positioned more distally in the bowel and the output remains stable upon initiation of oral feeding, a considerable number of patients can successfully tolerate enteral feeding. In either scenario, ensuring adequate nutrition is firmly established as a crucial and integral component in the management of ECF [4].
Accurate identification of the fistula and effective regulation of its output are pivotal steps in achieving patient stabilization. It is crucial to contain the leaked contents to prevent enzymatic harm to the surrounding skin and facilitate the healing process. Implementing appropriate wound care measures not only prevents skin deterioration but also reduces discomfort, thereby enhancing the patient's overall quality of life [5].
Once the patient's condition is stabilized, determining the most suitable course of action for definitive treatment of the fistula becomes a priority. Although a significant proportion of fistula cases are managed non-operatively, immediate surgical intervention may be warranted under certain circumstances [6].
In terms of timing, a watchful waiting period of 4 to 6 weeks is often employed before considering surgical options, contingent upon the patient's stability. Spontaneous closure of a majority of ECF cases occurs within approximately 5 weeks. This approach not only contributes to reducing the morbidity and mortality associated with relaparotomy but also emphasizes medical treatment as the cornerstone during this phase, while simultaneously addressing all modifiable factors. Notably, low-output fistulas tend to close at a faster rate compared to those with higher output, and a lengthier fistula tract correlates with a higher likelihood of closure [7,8].
Medical therapy plays a crucial role in mitigating fistula outflow, thereby promoting the potential for spontaneous closure. It is imperative to avoid the use of nasogastric tubes whenever possible. In instances of high-output fistulas, Proton Pump Inhibitors (PPIs) and H 2 blockers can be administered to curtail gastric secretions [9]. Furthermore, antidiarrheal medications such as loperamide have proven effective in reducing the output of high-output ECF cases [10]. Among the medical interventions, the utilization of somatostatin analog (octreotide) has been extensively studied for regulating fistula output. Its application has demonstrated the ability to decrease fistula output, expedite spontaneous closure, and lead to shorter hospital stays. However, it is important to note that octreotide has not shown the ability to reduce mortality rates [11].
When medical interventions fail to sufficiently reduce fistula output, surgical options become a consideration. Nonetheless, surgical closure during the inflammatory phase poses considerable challenges, with a significant risk of recurrence. Delicate handling of the bowels and meticulous excision of the fistula tract is essential, particularly to prevent inadvertent enterotomies during processes like adhesion lysis and bowel mobilization [12,13].
In the absence of inflammation, the optimal approach involves excising the fistula tract and resecting a short segment of the associated bowel, followed by surgical anastomosis to restore intestinal continuity. To reduce the likelihood of recurrence, it is imperative to close the fascia through which the fistula tract traversed. Achieving a permanent resolution for ECF is attainable in 80% to 95% of cases with appropriate medical care, proper diet, and a suitable waiting period before surgery [4].
Approximately 68% of ECF cases close spontaneously with enhanced healthcare and adequate nutrition [14,15]. Mortality and the absence of spontaneous closure are more prevalent in scenarios involving cancer operations, inflammatory bowel disease procedures, or adhesion lysis [16].
Despite the numerous uncertainties surrounding ECF in our context, no recent studies have been conducted to address this issue. There is a glaring absence of data on the predictors of management outcomes for ECF patients in our setting, regardless of the number of admissions or fatalities. This study aims to bridge this knowledge gap by examining the factors associated with management outcomes of patients with enterocutaneous fistula who receive care at the University of Dodoma Affiliated Teaching Hospitals.
In this 12-month hospital-based longitudinal analytical study, 60 patients with ECF were recruited from August 2022 to July 2023 at the Benjamin Mkapa Hospital, Iringa Regional Referral Hospital, and Dodoma Regional Referral Hospital in Tanzania. This study comprised patients with ECF who were admitted to general surgery departments and who had or did not have background of abdominal surgery background.
A non-probability convenience sample was used because of the way the hospital-based study was set up and the possible scarcity of ECF patients who might be enrolled. The accessibility of patients inside the teaching hospitals affiliated to the University of Dodoma allows for the viability and practicability of this strategy. Exclusion criteria included the following: a) Patients whose medical records were insufficient to accurately assess their medical history, diagnosis, and course of treatment; b) Pregnant women due to potential complications associated with their condition; c) Patients with severe comorbid conditions or life-threatening illnesses.
Important demographic data, clinical history, diagnostic information, treatment modalities, and subsequent results pertinent to patients presenting with ECF were thoroughly gathered using a painstakingly crafted questionnaire. The following variables were analyzed: the patient's age, electrolytes, albumin level, hemoglobin level (Hb), glycated hemoglobin (HBA1c), CD4 count for HIV-positive patients, surgical and non-surgical treatment options [total parenteral nutrition (TPN), medication, and watchful waiting], number of hospital days, and death.
The structured questionnaire was used in face-to-face interviews by trained research staff with qualified patients. To guarantee the precision and comprehensiveness of the responses, these interviews took place on hospital grounds. The many components of the questionnaire served as a guide for the interview procedure, which carefully recorded standardized data from each participant. These interviews were conducted within a confidential and serene environment to ensure participant comfort and information confidentiality.
On the other occasion medically trained members of the research team retrieved pertinent clinical information from patients' medical records. During the data collection process, patients with ECF and concurrent comorbidities like autoimmune illnesses, HIV/AIDS, diabetes mellitus, hypertension, cardiovascular diseases, renal disorders, and malnutrition were questioned. Utilizing the International Classification.
Millimolar per liter (mol/l) units were used to express laboratory measurements of electrolyte levels. Patients who were stable underwent these measurements at least once, but those who were unstable or displaying signs of derangement underwent them serially. Glycated hemoglobin readings, given as a percentage (%), were used to calculate blood sugar levels. Milligrams per deciliter (mg/dl) were used to measure hemoglobin concentration. Assessments of CD4 counts were only performed on HIV-positive individuals, and the results were expressed in cells per deciliter of blood. Milligrams per deciliter (mg/dl) measurements of serum albumin levels were made.
Upon the diagnosis of ECF for each patient, the systematic monitoring of these laboratory tests was initiated. This thorough surveillance was continued throughout the patient's hospital stay and diligently continued into the post-discharge follow-up phase. Careful records of the laboratory results were kept during the data entry procedure in terms of the average values of each test. By ensuring correctness and precision in the assimilation of laboratory data, this method facilitated efficient analysis and interpretation of these crucial clinical indicators.
Both surgical and non-surgical management options were offered. The option of stoma formation was available in situations where permanent closure of the fistula was not possible and cases needing assisted surgery to permanently close the fistula (such as an anastomosis) were deemed surgical treatments. Non-surgical options included watchful waiting (carrying on with postoperative care already in place) and medical intervention. Some of the medications used to treat ECF symptoms or delay the disease's course include proton pump inhibitors (PPIs), antibiotics, analgesics, intravenous fluids, parenteral nutrition, and somatostatins.
There were two categories of patient outcomes: Favorable (early home discharge within 28 days without complications) and unfavorable (long hospital stay or fatality). Outcomes ECF were assessed during hospitalization and for a maximum of six weeks after discharge.
The SPSS version 26 was used to generate and thoroughly analyze the acquired data. A thorough data cleaning process was started in order to guarantee data accuracy and integrity. Examining outliers, consistency, missing values, and frequency distributions across variables were all part of this process. To improve the accuracy of the dataset, any discrepancies or anomalies were found and fixed.
The Chi-square test was used in order to evaluate the variables affecting management outcomes. Following the Chi-square analysis, variables that showed statistically significant relationships were re-examined. Univariate logistic regression was performed, followed by a multivariate logistic regression analysis. These progressive analyses permitted the identification of variables that independently correlated with management outcomes. A p-value of less than 0.05 was used as the criterion for statistical significance.
Prior to the commencement of this study, the necessary ethical clearance was diligently obtained from the University of Dodoma Institute of Research Review Committee (IRRC). This crucial step ensures that the research adheres to established ethical guidelines, safeguarding the rights, well-being, and confidentiality of the study participants. Furthermore, the requisite permissions to conduct the study within Benjamin Mkapa Hospital, Dodoma Regional Referral Hospital, and Iringa Regional Referral Hospital were sought. These permissions were secured through direct communication with the respective hospital directors and medical officers in charge of each institution. Throughout the course of the study, confidentiality of the subjects was well maintained as numbers were used instead of names.
Sixty patients with ECF were recruited in this study; the mean age of the study participants was 40.3 ± 14.5 years ranging from 13 to 68 years. Majority of the patients (38.3%) were in the age group of 45 years and above. A little over half (55%) were women, nearly all patients (98.3%) had undergone abdominal surgery previously, and they also had hypoalbuminemia (31.7%), hyponatremia (30.0%), hypokalemia (25.2%) and anemia. Also, (1.7%). 8.3% of people had HIV, 10% had diabetes mellitus, and just 3.3% had hypertension, according to the statistics on chronic illness as shown on Table 1.
Table 1: Socio-demographic and clinical characteristics of the study population. View Table 1
Only 18% of all patients had favorable outcomes which included spontaneous closure without any complication and all those who had short duration of hospital stay and the rest 82% had unfavorable outcomes as shown on Figure 1.
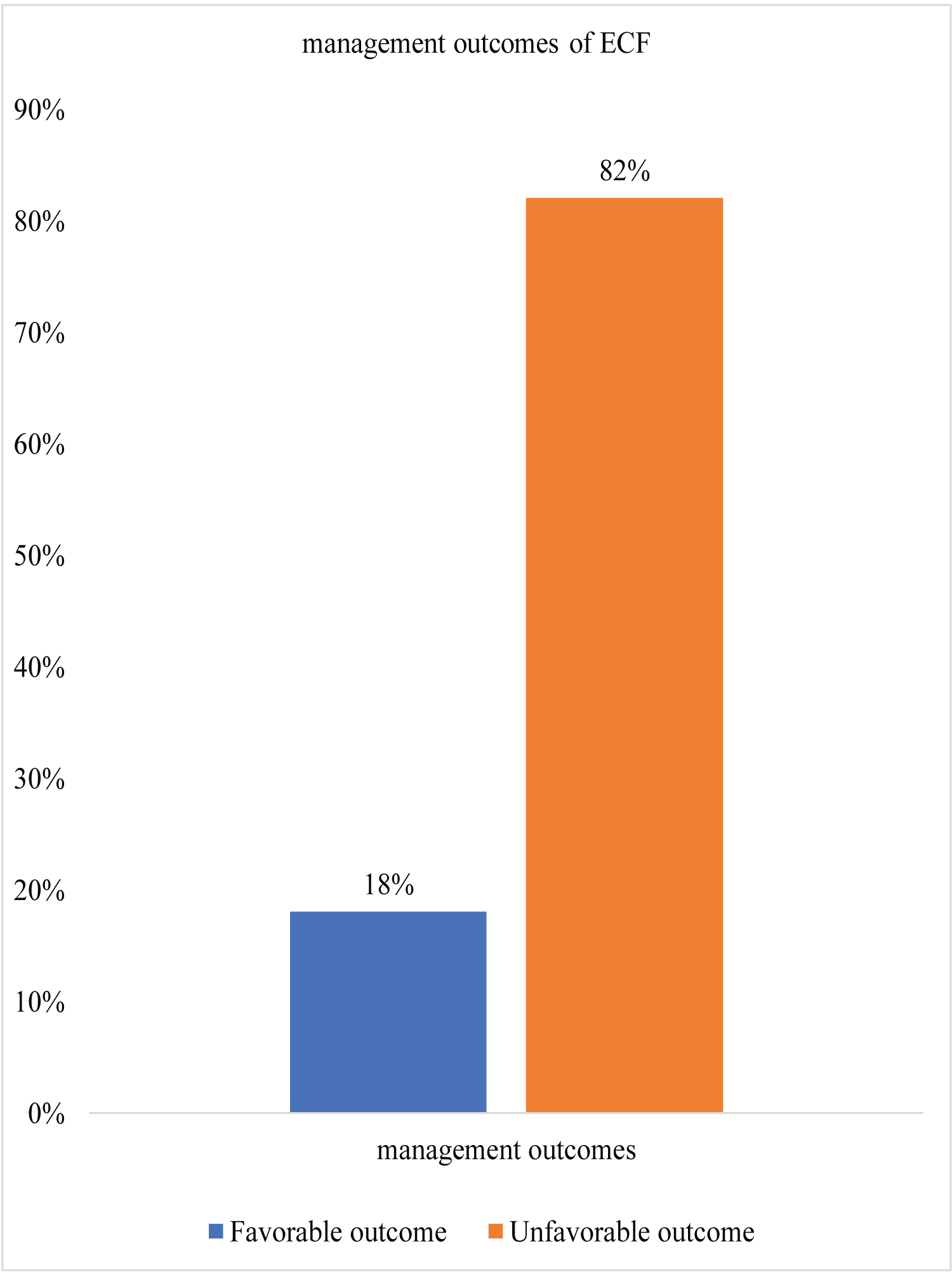 Figure 1: Overall ECF outcomes among study participants.
View Figure 1
Figure 1: Overall ECF outcomes among study participants.
View Figure 1
About 68.3% of all patients had unsuccessful fistula closure; the 31.7% that closed successfully, did it spontaneously. The mean duration of spontaneous closure was 27.7 ± 9.4 days. About 38.3% of patients with unsuccessful closure underwent stoma formation; and 17.7% proceeded past within 6 weeks without spontaneous closure. Mortality was 30%. Majority 73.3%, of survivors stayed in the ward for more than 28 days (Figure 2).
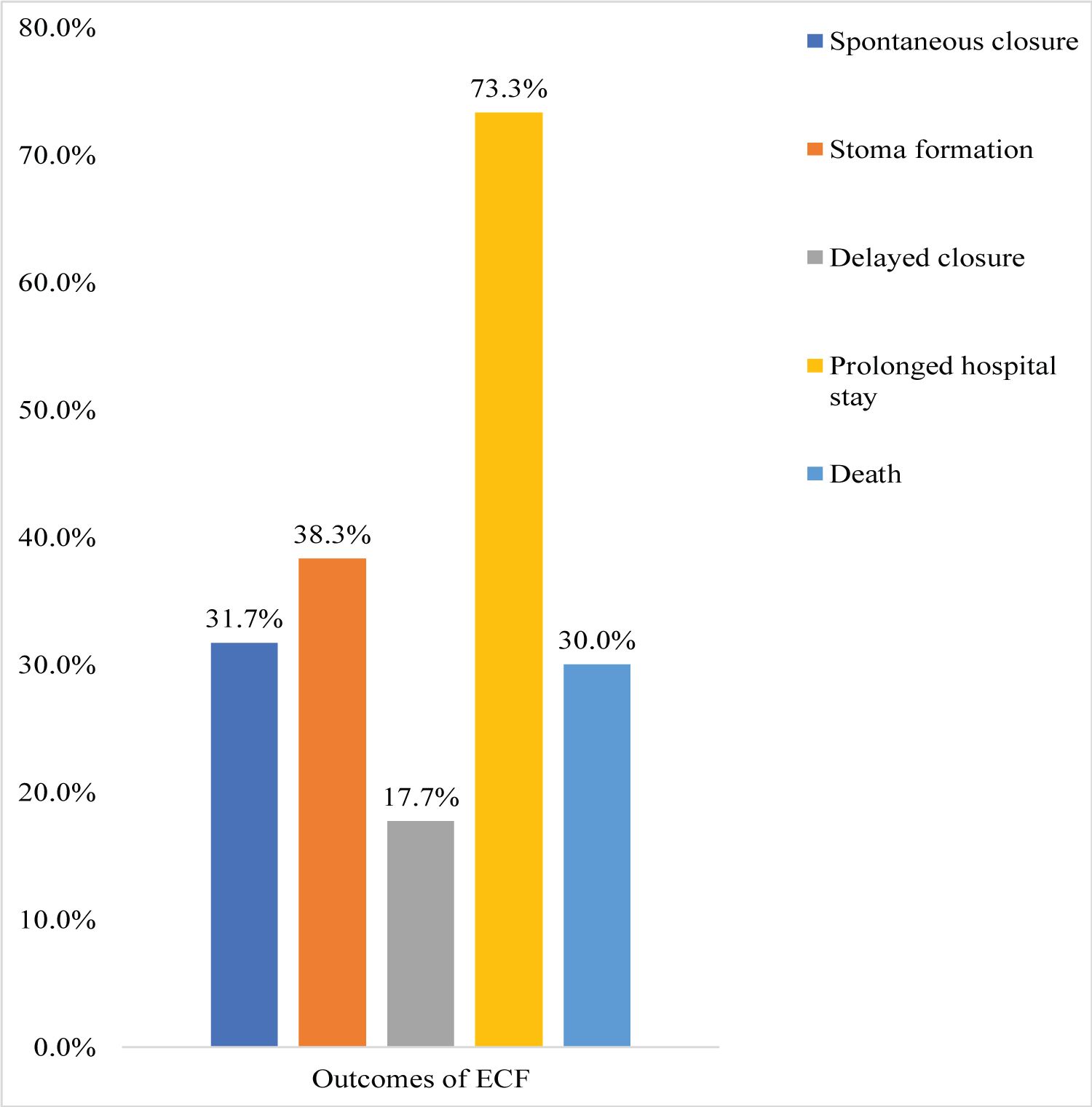 Figure 2: Distribution of outcomes among the study participants.
View Figure 2
Figure 2: Distribution of outcomes among the study participants.
View Figure 2
In multivariate logistic regression analysis as shown in Table 2 below, patients with age of ≥ 40 years presented with 24 (95% CI, 268-457.533) times higher risk of undesirable patients’ outcomes compared to other factors with p value of 0.034 which shown statistical significance. Also, low level of hemoglobin [AOR 6 (95% CI, 1.115- 32.42)] and small bowel ECF [AOR 18 (95% CI, 1.311-260.562)] were statistical associated with undesired patients’ outcomes with p value of 0.037 and 0.031 respectively. The findings demonstrate that patients presented with low level hemoglobin were at risk of getting undesirable outcomes six times compared to other factors and those with small bowel ECF were at risk of having undesired outcomes 18 times compared to other variables Table 2.
Table 2: Binary logistic regression of factors associated with outcomes of patients with enterocutaneous fistula (probability was set for unfavorable outcomes to occur). View Table 2
In the present study, we observed spontaneous closure in 31.7% of cases, with an average duration of closure recorded at 27.7 ± 9.4 days. Similarly, spontaneous closure of the fistula occurred in 30% of cases, on average after 25.6 days [17]. However, the rate of spontaneous closure appeared to be lower when compared to a previous study conducted in Tanzania, which reported a higher rate of 45.7% for spontaneous closure of enterocutaneous fistulas (ECF) [18]. Notably, a significant disparity between these studies emerged in terms of the duration required for closure, with our study indicating an average closure time of 27 days, while the study by Chalya, et al. documented an average duration of 50 days for ECF closure.
The literature has reported a wide range of rates for spontaneous closure of fistulas, spanning from 23% to 80% [19,20]. Some studies have suggested that fistulas resulting from surgical cause exhibit higher rates of spontaneous closure when compared to those stemming from non-surgical causes. These studies have concluded that the likelihood of spontaneous closure is elevated in surgical fistulas without complications. However, in our study, nearly all the observed fistulas were a result of post-surgical complications, making it challenging to fully endorse this notion due to the absence of a comparative group. Nevertheless, it is noteworthy that our study did reveal a relatively high rate of spontaneous closure among these cases.
Within our study, a mortality rate of 30% was observed, mirroring the findings of a study conducted in Tanzania by P. L. Chalya, et al. in 2010, which reported a comparable mortality rate of 30.4% [18]. Notably, despite the passage of a decade between these studies, the similarity in mortality rates suggests a lack of discernible improvement in the management of ECF within the Tanzanian context. This mortality rate is high but within a range of 6% to 33% observed in a meta-analysis published in 2012 [17]. However, experts advise early identification and fast infection control, fistula path redirection, electrolyte and nutritional support methods are said to minimize complications and mortality [17].
This phenomenon is not unique to Tanzania, as mortality rates of a similar magnitude have been observed in various other regions. For instance, studies conducted in Cameroon revealed a mortality rate of 38.6% [21], aligning with findings from Muhindo's study in Congo and studies in Nigeria, each reporting mortality rates exceeding 30% and studies conducted in India documented a mortality rate of 29% [19,21-24]. These cumulative observations underscore the persistent challenges in managing ECF and the need for concerted efforts to improve patient outcomes across different geographical contexts. In contrast, lower mortality rates were reported in settings such as Switzerland, Spain, and the UK, where the observed rates were all under 10% [25,26].
This discrepancy may be attributed to the more affluent healthcare environments and advanced medical technology platforms available in those regions [27-30]. In settings like ours, the situation differs markedly. Patients often arrive for treatment at a later stage, and limited medical resources for resuscitation due to economic challenges can contribute to elevated mortality rates. The presence of poverty within our context potentially exacerbates these challenges, leading to the observed higher mortality rates. This further underscores the critical role socio-economic factors play in shaping healthcare outcomes.
In our research, we observed that age above 40 years, the presence of small bowel enterocutaneous fistulas (ECF), and lower hemoglobin levels were all identified as statistically significant predictors of unfavorable outcomes. These findings align with previous studies conducted in various regions, including Tanzania [18], India, Nigeria, Iraq, Cameroon, and Guinea [12,20,21,24,31-38], where similar predictors with addition of comorbidities like HIV and diabetes mellitus were also reported as contributing factors to poor ECF outcomes. It's crucial to remember that small bowel fistulas typically have a high output, which can cause electrolyte imbalances and hypoalbuminemia because of the natural fluid leakage of fistulas and the frequently noticed impaired nutritional status in ECF patients [39]. With regards to age, older people may have decreased immune systems, slower healing times, a higher prevalence of chronic diseases, and malnutrition, which might impede the management of ECF and recovery. Anemia, which is indicated by low hemoglobin levels, can have a serious negative effect on ECF patients' prognoses. Anemia can make it more difficult to treat ECF since it can slow down tissue healing, raise the risk of infection, signify poor nutritional condition, reduce physical stamina, and delay wound healing.
Given these results, it is critical for medical personnel to identify and take these important variables into account when treating patients with ECF. To improve the prognosis and standard of care for patients with ECF, a holistic and multidisciplinary approach that considers age, the specific type of fistula, hemoglobin levels, and the presence of comorbidities is crucial.
Despite advancements in management, the mortality rate for enterocutaneous fistulas remains alarmingly high at 30%. Statistical analyses reveal that high-output ECF, age above 45 years and anemia stand as significant predictors of unfavorable outcomes. These findings underscore the multifaceted nature of managing enterocutaneous fistulas and the importance of addressing both the primary condition and its associated complications to improve patient outcomes.
Meticulous attention should be directed towards managing small bowel ECF and anemia which are significant modifiable predictors of mortality. Adequate electrolyte and nutritional support, including nutrition supplementation especially TPN and fluid management, should be intensively integrated into the treatment plan.
Given the intricate interplay between various factors affecting ECF outcomes, further research is warranted to delve deeper into the underlying mechanisms and potential interventions, aiming to reduce mortality rates and enhance patient care.
We appreciate the help we received from Dr. Frank Sandi, Head of the Department of General Surgery at the University of Dodoma, and the other faculty members, especially Dr. Edward Msokwa. They helped shape the hypothesis through their insightful discussions and contributed to the overall idea of this dissertation.
Moreover, we would like to express our gratitude to all of the general surgery staff at Benjamin Mkapa Hospital (BMH), Iringa Regional Referral Hospital (IRRH), and Dodoma Regional Referral Hospital (DRRH) for their guidance and support throughout the whole data collection period.
Prior to the commencement of this study, the necessary ethical clearance was diligently obtained from the University of Dodoma Institute of Research Review Committee (IRRC). Furthermore, the requisite permissions to conduct the study within Benjamin Mkapa Hospital, Dodoma Regional Referral Hospital, and Iringa Regional Referral Hospital were sought. These permissions were secured through direct communication with the respective hospital directors and medical officers in charge of each institution. All study participants were requested to sign a written consent form and throughout the course of the study, confidentiality of the subjects was well maintained as numbers were used instead of names.
The author declares no competing interest.
None.