Prostate cancer treatment is associated with side effects including urinary incontinence, erectile dysfunction, fatigue, depressive symptoms, and a decrease in physical function. Physical exercise has been considered beneficial in countering these side effects. We believe that supervised exercise programs may be an efficient alternative to treatment and rehabilitation in these patients. As part of an ongoing analysis, we sought to evaluate a 12-week physical exercise protocol in prostate cancer survivors. Patients will be divided into two groups (intervention and control) and data were collected before and after the study. We hope to identify whether physical exercise is effective in prostate cancer survivors, and establish recommendations for specific exercises, nowadays this measures are so restricted especially.
Prostate neoplasm, Physical exercise, Exercise program, Protocol, Cancer
Prostate cancer has become a health public concern [1]. It is the sixth most common cancer in the world, the most prevalent cancer among men, and represents approximately 10% of all cancers [2]. In 2016, it was estimated that 295,200 men in Brazil had cancer, including 61,200 with prostate cancer [1]. Owing to advances in the detection and medical treatment of this condition, patients' survival rate has steadily increased [3]. However, a large proportion of prostate cancer survivors have side effects associated with the disease and its treatment, including urinary incontinence, erectile dysfunction, fatigue, depressive symptoms, anguish, and a decrease in physical function [4-6]. Fortunately, studies have demonstrated that regular physical exercise can minimize these side effects [7].
Current literature emphasizes the importance of regular physical activity in the general population [8,9]. Physical activity has also been shown to be beneficial in patients with cancer who have psychological and physical concerns [7]. At least 150 minutes of moderate or 75 minutes of intensive physical activity per week is recommended in patients with cancer [8]. The American College of Sports Medicine (ACSM) has similar recommendations and further suggests that resistance training be performed twice a week to improve general physical health [10]. In addition, researchers have suggested a combination of resistance and aerobic training in men with prostate cancer [11], because these patients tend to have treatment-related muscle loss, cardiorespiratory decline, urinary incontinence, and muscular fatigue.
However, data on physical activity protocols in patients with cancer are lacking. The only physical activities that have been evaluated include walking [12] and aerobic exercise combined with resistance training [13-15]. In addition, some studies have assessed behavioral support [16-18] and pelvic floor training [19]. Functional training is one of the modalities that combine resistance training, aerobic exercise, and pelvic floor training to improve people's functional capacity, daily activities, autonomy, and independence [20].
It is possible that this kind of activity is the most effective intervention in patients with prostate cancer. Previous researchers have reported deterioration of physical fitness associated with cancer and its treatment [2], decreasing muscle mass and strength, increasing weight and body fat, and decreasing in the physical functions [3]. Changes that could suffer improvement with regular practice of functional training [20], because it is based on variables of physical fitness provide a global evolution of the individual. In addition, physical exercises should include pelvic floor training to treat the urinary incontinence, which is associated with radical prostatectomy surgery, the oldest and possible more efficient measure to treat local prostate cancer [21].
In this study, we will be conducting a non-randomized clinical trial to evaluate the effectiveness a 12-week functional training protocol consisting of twice weekly exercise in an Experimental Group (EG) of patients with prostate cancer, and compared it to a Control Group (CG) of patients who did not perform any physical exercise. We hypothesize that patients in the EG will have improved quality-of-life, sexual activity, and physical fitness, as well as less fatigue and depression-related symptoms, compared with the CG.
Non-randomized clinical trial with a EG undergoing a 12-week intervention period and a CG.
Patients diagnosed with prostate cancer currently undergoing treatment or who have completed treatment will be included in the non-probabilistic sample.
Patients between 50-80 years will be included in the study. This age range was selected because it has the highest prevalence of prostate cancer, and patients are undergoing adjuvant or neoadjuvant treatment, or have completed treatment. Criteria for exclusion will include patients who are illiterate and those whose disease metastasized. The latter criterion was selected because of the variability of local metastases as it relates to treatment and prognosis. Furthermore, patients who exercised three months before the beginning of the study will be excluded from the analysis.
We estimated that 38 patients will be needed in the study based on sample size calculations using G*Power 3.1.9.2, with effect size of 0.67, level of significance of 5%, power of the test of 90%, and sample loss of 20% [22]. Patients will be divided into two groups: The CG (n = 19) and the EG (n = 19). Data will be collected before, during, and after the 12-week study period; patients will also attend lectures twice a week. Patients in the EG will undergo a 12-week functional training intervention, and be invited to continue the protocol after the conclusion of the study.
The protocol will be executed in a fitness center that focuses on functional training located in the mainland area of Florianopolis. We will use the local press to raise awareness about the study and recruit patients. Patients will also be recruited from the "Centro de Pesquisas Oncologicas" (CEPON) Oncological Research Center, in Florianopolis. Patients will be contacted by phone and invited to participate in the study. Procedures including prior collection of data, application of the functional training, and post-study data collection will be explained; the importance of the assiduity will also be discussed. Once patients agree to follow the study protocol, they will be asked to sign a consent form, after which point data collection will begin. The initial assessment will consist of a quiz (Table 1) and physical fitness test (Table 2). Both will occur one week before the intervention at a silent location to ensure that patients understand the quiz. The intervention will be initiated after the initial data collection, and last one hour twice weekly in accordance with the study protocol. The same quiz and physical fitness test will be administered at the conclusion of the 12-week intervention.
Table 1: Functional training protocol for men diagnosed with prostate cancer. View Table 1
Table 2: Physical test. View Table 2
The protocol will include 24 different types of training. Functional exercise will comprise agility, balance, flexibility, resistance, potency, coordination, and strength. Each week, agility will be combined with another physical fitness variable. Each session will last one hour. Each session will consist of a 10-minute warm-up, followed by a 10-minute warm-up specific to an exercise, 30-minute of exercise, and a 10-minute cool down [7]. The first week of the intervention will focus on pelvic strength training, which will help improve the overall performance of the patients and urinary incontinence urinary. Aerobic activity will be performed through agility exercises during the warm-up session, whereas the main 30-minute exercise session will consist of resistance training. The warm-up and main exercise will be performed in cycles of 5 different types of exercises, where patients perform 8-12 repetitions for each circuit, 1 to 3 times, while respecting the physical limits of each patient.
Data in the CG will be simultaneously collected with the EG. The personal involved will be included in case of a lack of interest or possibility in being a part of the EG. We will schedule an at-home patient visit or meet with them at another location of their choosing. We will then thoroughly explain the purpose of the study, highlighting the importance of regular physical activity, and request that the patient does not initiate any physical activity similar to the intervention. To keep patients in the CG involved, they will attend biweekly lectures on topics including health maintenance, lesion prevention, and nutrition. Post-study data will be collected in patients who did perform physical activity during the study, but their data will be excluded from the analysis. The picture 01 shows the participants selection process and the execution of the study protocol (Figure 1).
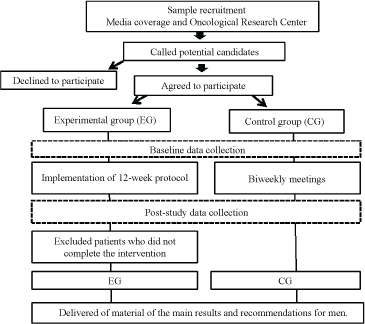 Figure 1: Flow chart of the selection process. View Figure 1
Figure 1: Flow chart of the selection process. View Figure 1
We will conduct biweekly meetings with the assistance of other professionals in the field of nutrition and physiotherapy. These meetings will be scheduled in advance and conducted at the same location as the intervention. They will last 30 minutes and consist of motivational lectures and basic practical guidelines for physical activity, including appropriated clothing and hydration.
A total of six meetings will be schedule; the topic of each meeting is listed in (Table 3).
Table 3: Meetings control group. View Table 3
Although validated protocols are lacking in this patient population most of the resistance training and aerobic exercises used in the intervention will already be familiar to the patients in our study and have been evaluated in previous studies [13-15,23]. We expect that the functional training intervention will provide positive physical and psychological results in this patient population (Table 4).
Table 4: Measures of results. View Table 4
An electronic spreadsheet will be made in Excel XP and data moved to the statistic package SPSS (IBM version 20.0). Descriptive statistics including average, standard curve (divert, diversion) and percentages will be calculated first so that we can understand the characteristics of the patient population. The Kolmogorov-Smirnov test will be used to determine the normal distribution of the outcomes; the normal will be set to p < 0.05. The average of the different outcomes will be calculated and compared between groups. Statistical differences will be obtained using variance analysis.
Univariate linear regression will be used to determine the β coefficient of each outcome and adjust for potential confounders in each group. Variables with p < 0.20 will be selected to produce a multi-linear regression model; p values < 0.05 will be considered statistically significant. The homoscedasticity will be analyzed and possible bias of the model through residual analyses, and all assumptions will be observed. Outcomes that do not present a normal distribution will be dichotomized in compliance with the theoretical reference or through central trend distribution measures if it is lacking. We will then perform a univariate logistical regression between groups to adjust for possible confounding variables. Variables with p values < 0.20 will be selected and adjusted in the multiple logistic regression models. Variables with p values < 0.05 and/or clinical significance will be maintained in the model.
The incidence of prostate cancer has been increasing since the 1960s and it has become a growing public health concern. According to the National Cancer Institute [1], we are more aware of this disease because of the remarkable advances in its diagnosis. As a result, there is a need to develop strategies assisting patients undergoing treatment and after treatment. Although physical activity has been shown to positively impact disease symptoms and treatment side effects [13-15,19], most cancer survivors are not physically active. Programs focusing on physical activity are needed to assist cancer survivors in adopting and maintaining a new life style. However, exercise programs are limited in this patient population. This is compounded by limited infrastructure and resources, lack of patient information and patient understanding of the benefits of physical activity, and a lack of recommendations to men with prostate cancer to perform physical activity [24].
These factors make it difficult to implement interventions in this patient population. Research is warranted to identify the possibilities and benefits from physical activity intervention programs in reducing disease- and treatment-related side effects in patients with cancer. To our knowledge, no study has been conducted evaluating functional training interventions to improve the quality of life of men with prostate cancer. In addition to decreasing fatigue and depression-related symptoms, physical activity improves self-esteem, sexual satisfaction, and fitness. Previous studies have evaluated walking protocols [12] and aerobic exercises combined with resistance exercises [23] in men with cancer. Other studies have evaluated interventions that were not validated, including walking [19], aerobic training [14,17], and pelvic floor exercise [19], as well as support groups to improve quality-of-life [13,16]. These studies evaluating functional training protocols have reported improvements in quality-of-life, life style, abdominal circumference, and general fatigue, as well as a decrease in arterial pressure and anxiety.
This non-randomized functional training protocol for men with prostate cancer was developed to encourage physical activity and decrease side effects associated with prostate cancer treatment. It includes challenging exercises, but in a safe and progressive way that combines aerobic and resistance exercise based on previous research [11]. We expect that the intervention will improve the study participants' quality-of-life, fatigue, strength, balance, coordination, body mass control, physical fitness self-esteem, and depression-related symptoms.
With the significant increase of survival in men with prostate cancer, new knowledge is needed on the impact of physical activity. An increase of the quality of life and other related factors in men with prostate cancer may lead to new public policies motivating them to be more physically active after their diagnosis. Research may also add a new perspective to the current body of evidence on this subject and contribute to an appropriate physical activity protocol for this population.
View Ethical Statement.