Amyotrophic Lateral Sclerosis (ALS) is a neurodegenerative disease, which signs of motor neuron dysfunction in one or more anatomic regions. New image processing methods have showed degeneration on grey and white matter of different cortical and subcortical brain structures.
Describe brain changes in a group of ALS patients through post-processing MRI techniques and correlate it with clinic abnormalities.
Thirty sporadic ALS patients and thirty healthy subjects were recruited, they were paired in age and sex. T1 and T2 weighted, FLAIR and DTI3T MRI scans were acquired. Tractography, Voxel Based Morphometry (VBM) and cortical thickness processing analysis were done.
In relation of ALS patients, tractography revealed that corticospinal tract number of fibers and diameter were lower than healthy subjects (p = 0.03); while optic radiation showed diminish of RD and increase of MD (p = 0.03). The number of fibers of evaluated tracts diminishes when disease duration increasing (p = 0.00) and when ALSFRS score is worse (p = 0.04). VBM revealed brain atrophy on cortical areas, cerebellum, brainstem, deep gray nuclei, corpus callosum, corticospinal tract, internal and external capsule, lower and middle longitudinal fascicle and optical radiation of ALS patients. Cortical thickness analysis showed diminish of thickness on bilaterally pre-central gyrus and parietal posterior areas (p = 0.03) on ALS patients. It diminishes when disease duration increase (p = 0.00) and when ALSFRS score is worse (p = 0.00).
In our ALS patient’s series was demonstrated morphologic abnormalities of grey and white mater of cortex, subcortical structures, cerebellum and brainstem. These abnormalities increase when clinical aspects get worse.
ALS, Tractography, Voxel based morphometry, Cortical thickness
According to the ICD-11, Amyotrophic Lateral Sclerosis (ALS, code: 8B60.0) is a progressive, fatal disorder in which signs of lower and upper motor neuron degeneration are seen in one or more regions: bulbar, cervical, thoracic or lumbosacral [1]. Median survival time is 2-4 years from onset of symptoms and it is the third more frequent neurodegenerative disease [2,3].
Electrophysiological studies should be required to confirm it.MRI, may be performed to exclude other causes that might explain clinical and electrophysiological features [2-5].
Incidence rate have been reported 2-3 per 100,000 person-years in European population, 0.7-0.8 in Asian population and 0.55 in Cuba, for that reason it is considered as a rare disease, but the number of patients is increasing [6].
Recently have changed the widely held belief that ALS affects only the motor neuron system, because evidence of new images methods suggest that ALS is a multisystem neurodegenerative disease that involves further sensory and extrapyramidal systems [1-3].
With this research we intend to describe the brain structural changes in a group of ALS patients through post-processing MRI studies and we propose correlate these changes with clinic abnormalities.
A total of 30 consecutive sporadic ALS patients were recruited between 2018 and 2020, according to the revised El Escorial criteria for clinically definite or probable [7]. The control group consisted of 30 healthy subjects.
No clinical diagnosis of frontotemporal dementia (FTD) and no cognitive impairment symptoms were found in these patients.
The study protocol was approved by Ethical Committee of Cuban Neuroscience Centre and all of patients and healthy subjects were agree with the evaluation and signed informed consent. All procedures of the study were in accordance with the ethical standards of the institution and with the 1964 Helsinki declaration.
Persons with contraindication for MRI and who deny participating.
ALS Functional Rating Scale Reviewed (ALSFRS-R) was used to evaluate the functional status of ALS patients. It is based on 12 items; scores range from 0 (maximum disability) to 48 (normal) points.
MRI was carried out using a 3T Allegra system scanner (Siemens) device with a standard quadrature head coil.
High-resolution three-dimensional whole-brain T1-weighted MRI scans were acquired using a volumetric three-dimensional spoiled fast gradient echo with the following parameters: Repetition time (TR) = 2000 ms, echo time (TE) = 2.6 ms, inversion time (TI) = 900 ms, slice thickness = 1.0 mm; flip angle = 9 Ê, field of view (FOV) = 230 × 230 mm, 1 × 1 × 1 mm3 voxel size. The volume consisted of 192 contiguous coronal sections covering the entire brain, acquisition matrix: 256 × 256.
T2-weighted MRI scans were acquired with TR = 3500 ms, TE = 354 ms.
FLAIR MRI scans were acquired with TR = 5000 ms, TE = 353 ms, TI = 1800 ms.
DWI data were acquired using a diffusion weighted spin echo imaging sequence with the following parameters: 80 volumes, slice thickness 1.0 mm, representing 80 gradient directions, FOV = 230 mm, TR = 86 ms, TE = 8000 ms; flip angle = 90, b = 1000 s/mm2 and two scan with gradient 0 (b = 0), resolution was 1 × 1 × 1.
DICOM to nifty image format was convert with dcm2nii tool, Chris Rorden's dcm2nii:4AUGUST2014 32bit (https://www.nitrc.org/projects/dcm2nii/).
DTI processing: DTI images were processed using DSI Studio software tool (https://dsi-studio.labsolver.org/).
Images were reoriented into oblique axial, slices were aligned parallel to the anterior-posterior commissural axis with the origin set to the anterior commissure, Eddy currents distortions were corrected, diffusion tensor was estimated, scalar maps were constructed, fibre tracking was done, tensor were visualized and tractography based analysis (automatic) was done.
Based on Montreal neurologic Institute (MNI) maps, ROIs was placed at left (cortico spinal tract, arcuate fascicle and optic radiation) with a volume size of 2.7e+04 mm cubic. An ROI was placed at right (cortico spinal_tract, arcuate fascicle and optic radiation) with a volume size of 2.9e+04 mm cubic. A seeding region was placed at whole brain. The anisotropy threshold was randomly selected. The change threshold was 20%. The angular threshold was randomly selected from 15 degrees to 90 degrees. The step size was randomly selected from 0.5 voxel to 1.5 voxels. Tracks with length shorter than 26.9531 or longer than 269.531 mm were discarded. A total of 50000 seeds were placed.
Once tracts were constructed, the following quantitative metrics were calculated automatically:
- Number of tracts (called too number of fibres or count of fibres): It is the number of streamlines generated by the algorithm (n).
- Tract length or mean longitude: It was calculated by multiplying number of coordinates in the streamlines with the distance between the coordinates. It was calculated trough the following equation:
n is the total number of tracks, vi(t) is a sequence of 3D coordinates representing the trajectory of a track, t is a discrete variable from 1 to mi, where mi is the number of the coordinates.
- Diameter (in mm): It was calculated trough the following equation:
- Irregularity: Is conceptually similar to convexity and concavity. It is the opposite of compactness or roundness defined in computer vision. It was calculated trough the following equation:
- Fractional Anisotropy (FA): It is a scalar measure of the preferential axis of diffusion motion. It is related to the integrity of the myelin, the density and the parallelism of the fibres, it has a value ranged from 0 (isotropic) to 1 (totally anisotropic).
- Mean Diffusivity (MD): It is the average displacement of water within a voxel in the main axes.
- Axial Diffusivity (AD): It quantifies how fast water diffuses along the axonal fibres. It is low on axonal damage.
- Radial Diffusivity (RD): It evaluates the perpendicular component of water diffusion to axons [8].
VBM was a fully automated, whole-brain technique that enables measurement of regional brain volumes based on voxel-wise comparison of grey and white matter volumes and the DARTEL registration method; it was done using Statistical Parametric Mapping8 software, running on Matlab 2017a.
Grey (GM) and white matter (WM) of ALS patients and healthy subjects were compared using t-test statistical analysis, with p < 0.05.
Computational Anatomy Toolbox (CAT), version CAT12.6-rc1 (1430) was used, it runs within SPM12. This software tools are freely available at http://dbm.neuro.uni-jena.de/cat/.
CAT uses a fully automated method that allows the measurement of cortical thickness and reconstruction of the central surface. It uses a tissue segmentation to estimate the WM distance and then projects the local maxima onto other GM voxels using a neighbouring relationship described by the WM distance [9,10].
Descriptive statistical measures were applied.
Between groups mean comparison (t-test) was done to compare mean parameters of tractography of ALS and healthy subjects.
Regression analysis was done in order to evaluate the relation of number of fibres of all of analysed tracts and mean cortical thickness value with disease duration and with ALSFRS-R score in ALS patients.
By group automated analysis (based on t-test) was done for VBM and cortical thickness, maps of signification were construed.
We applied a statistical threshold of p < 0.05 in all statistical analysis.
Clinic details of all of participants could be seen on Table 1.
Table 1: Clinic characteristics of ALS patients and healthy subjects. View Table 1
Comparison of tractography parameters between groups (ALS patients and healthy subjects): Corticospinal Tract (CST) statistical analysis
It showed diminish of mean number of tracts (p = 0.03) and diameter (p = 0.03) of ALS patients in relation with healthy subjects (See Table 2 and Figure 1).
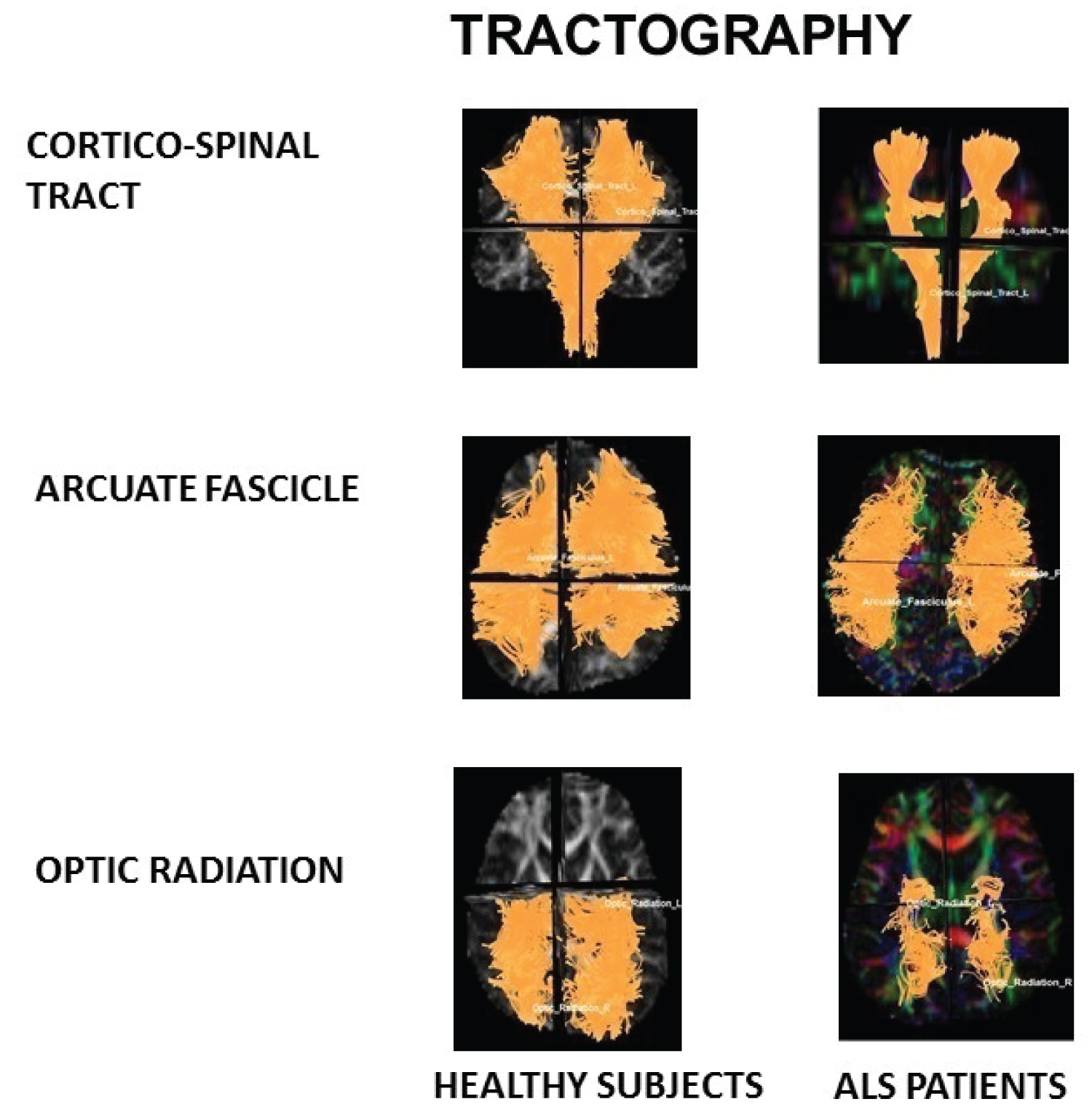 Figure 1: Tractography.
Figure 1: Tractography.
Note diminish of fibres number apparent on cortico-spinal tract and optic radiation on ALS patients in relation with healthy subjects.
View Figure 1
Table 2: Mean comparison of CST parameters between healthy subjects and ALS patients. View Table 2
Arcuate Fascicle (AF) statistical analysis
It showed that any mean parameters were statistically significant to comparison between ALS patients in relation with healthy subjects.
Optic Radiation (OR) statistical analysis
It showed diminish of RD (p = 0.03) and increase of MD (p = 0.03) of ALS patients in relation with healthy subjects (See Table 3).
Table 3: Mean comparison of OR parameters between healthy subjects and ALS patients. View Table 3
Regression analysis
Analysis of number of tracts in relation with disease duration
It showed that number of tracts of CST, OR and AF diminishes when disease duration increasing (p = 0.00) (See Table 4a).
Table 4a: Regression summary for dependent variable: Number of tract on ALS patients in relation with (a) disease duration. View Table 4a
The rest of parameters of tractography of all evaluated tracts were not statistically significant in relation with the increase of the disease duration.
Analysis of number of tracts in relation with ALSFRS score
It showed that number of tracts of CST diminishes when ALSFRS score is worse (p = 0.04) See Table 4b.
Table 4b: Regression summary for dependent variable: Number of tract on ALS patients in relation with (b) ALSFRS-R score. View Table 4b
The rest of parameters of tractography of CST tracts were not statistically significant in relation with ALSFRS score.
Any parameters of tractography of OR and AF were statistically significant in relation with ALSFRS score.
Patterns of brain atrophy in grey matter: We observed reduced grey matter density on ALS patients in relation with healthy subjects on cingulate gyrus, anterior portion of occipital lobe, paracentral lobe, precuneus, cerebellum, uncus, gyrus parahipocampallis, gyrus lingual, medulla oblongata, tectum mesencephalic, frontal and parietal lobes, insula, claustrum, thalamic nucleus, globepallidum andputamen nucleus and amygdalin nucleus (Figure 2).
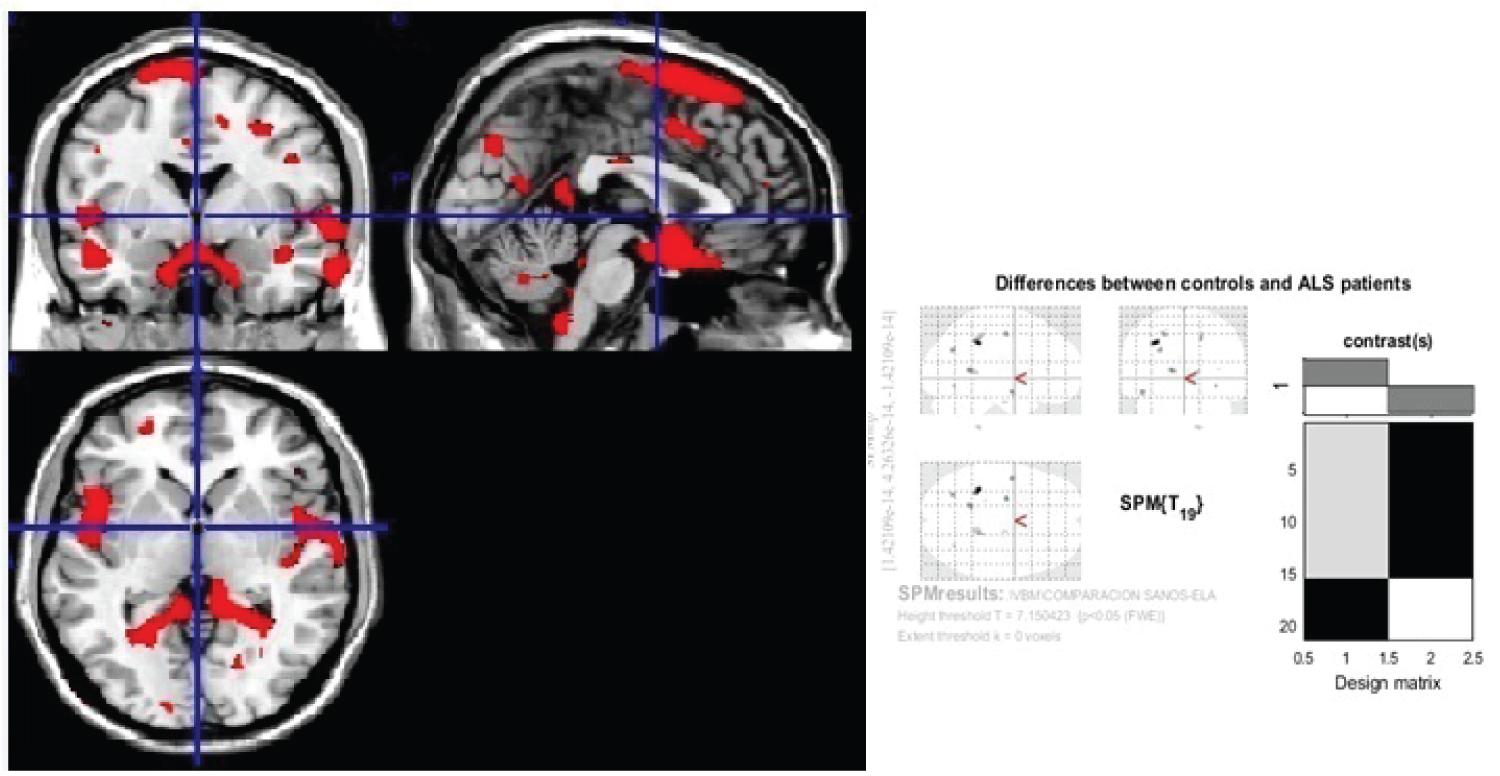 Figure 2: Generic MRI brain slices with superimposed areas showing statistically regions of grey matter atrophy (p < 0.05, corrected for multiple comparisons) in a group of 30 ALS patients compared with 30 healthy agematched subjects using voxel-based morphometry.
Figure 2: Generic MRI brain slices with superimposed areas showing statistically regions of grey matter atrophy (p < 0.05, corrected for multiple comparisons) in a group of 30 ALS patients compared with 30 healthy agematched subjects using voxel-based morphometry.
It shows grey matter atrophy on cingulate gyrus, anterior portion of occipital lobe, paracentral lobe, precuneus, occipital areas, cerebellum, uncus, gyrus parahipocampallis, gyrus lingual, medulla oblongata, tectum mesencephalic, frontal and parietal lobes, insula, claustrum, thalamic nucleus, globe palladium, putamen and amygdalin nucles. The colored bard represents the T score.
View Figure 2
Patterns of brain atrophy in white matter: We observed reduced white matter density in ALS patients in relation with healthy subjects on corpus callosum, corticospinal tract (at midbrain, pons and medulla oblongata), internal capsule and external capsule, optical radiation, lower and middle longitudinal fascicle, white matter of frontal, parietal and occipital areas (Figure 3).
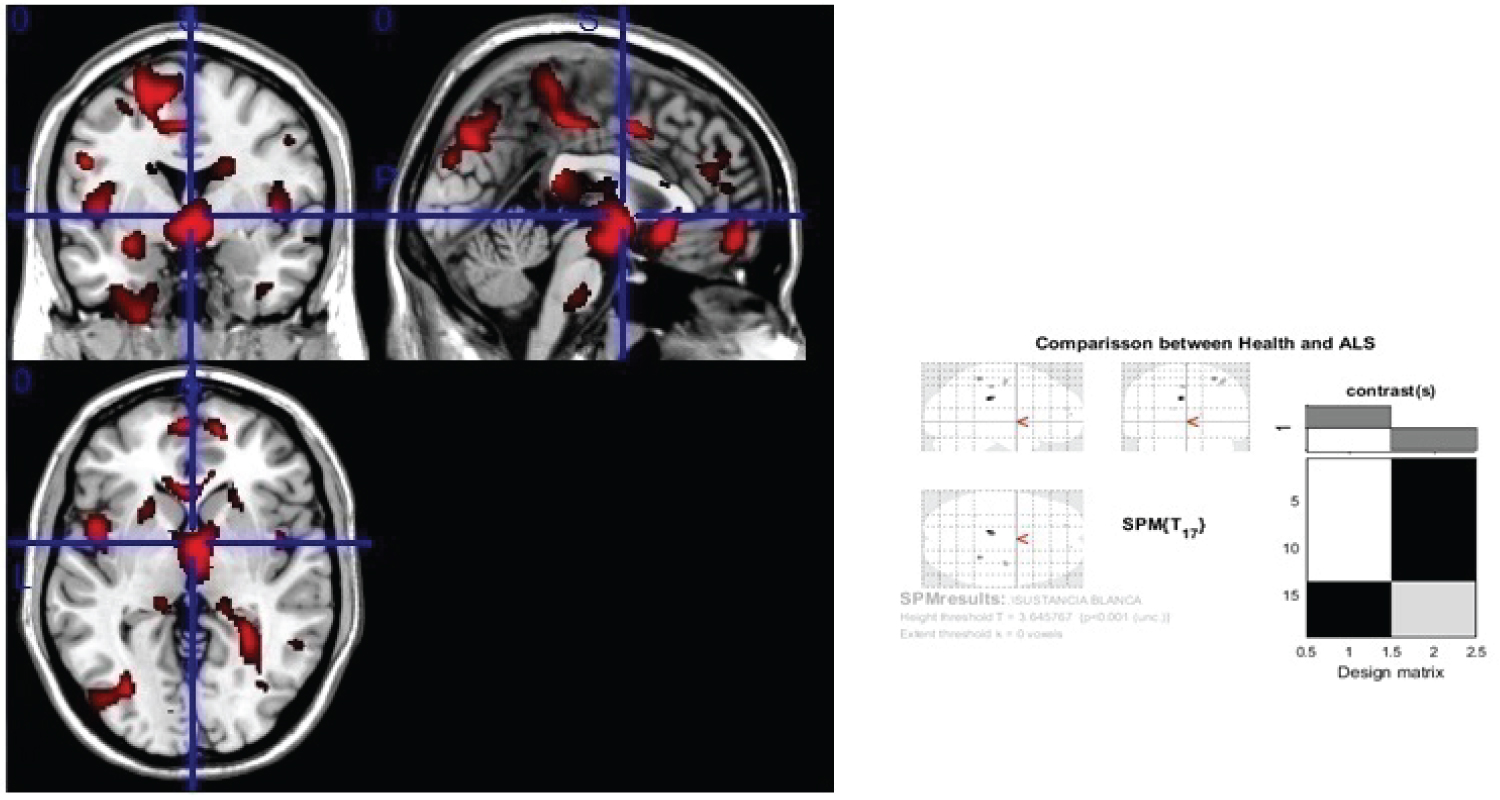 Figure 3: Generic MRI brain slices with superimposed areas showing statistically significant regions of white matter atrophy (p < 0.05, corrected for multiple comparisons) in a group of 30 ALS patients compared with 30 healthy agematched subjects, using voxel-based morphometry.
Figure 3: Generic MRI brain slices with superimposed areas showing statistically significant regions of white matter atrophy (p < 0.05, corrected for multiple comparisons) in a group of 30 ALS patients compared with 30 healthy agematched subjects, using voxel-based morphometry.
It shows white matter atrophy on corpus callosum, corticospinal tract (at midbrain, pons and medulla oblongata), internal and external capsule, optical radiation, lower and middle longitudinal fascicle, white matter of frontal, parietal and occipital areas. The colored bar represents the T score.
View Figure 3
Comparison between groups (ALS patients and healthy subjects): ALS patients showed diminish of mean thickness on bilaterally pre-central gyrus and parietal posterior areas (p = 0.03) See Figure 4.
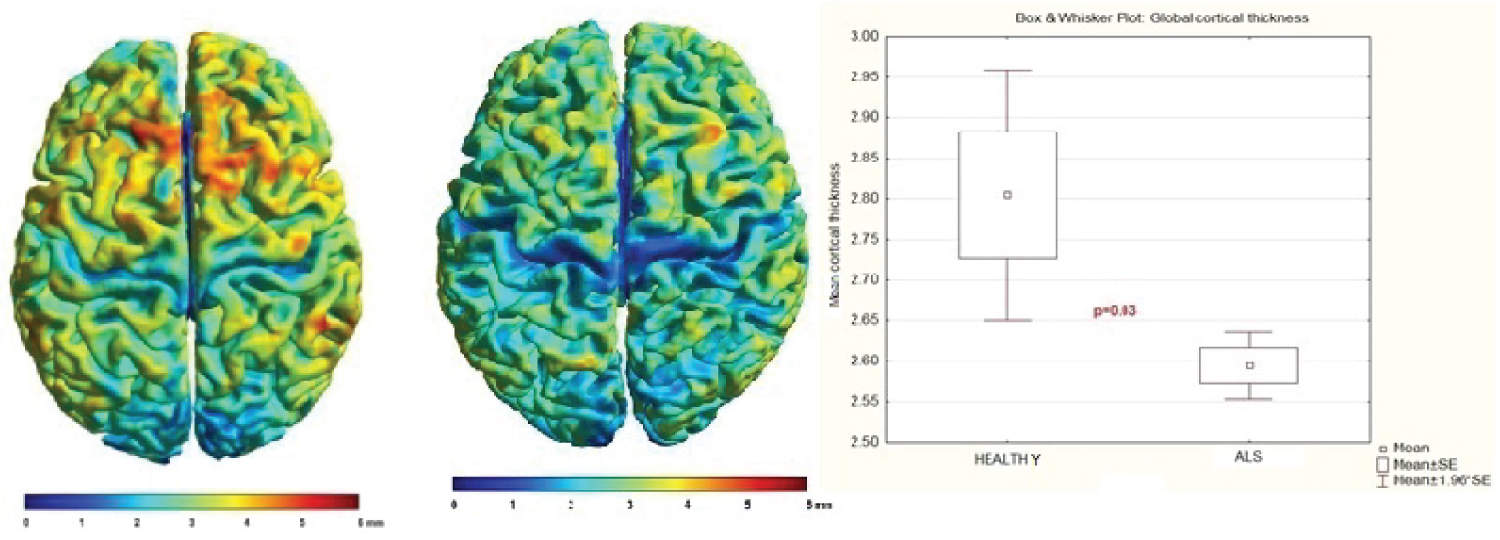 Figure 4: Comparison of individual mean cortical thickness between healthy subjects and ALS patients.
Figure 4: Comparison of individual mean cortical thickness between healthy subjects and ALS patients.
Notice it is diminished in ALS patients. p = 0.03
View Figure 4
Group statistical analysis of cortical thickness showed differences between ALS and healthy subjects: ALS patients had diminished of cortical thickness on parietal area and posterior and superior sites of frontal lobe in relation with healthy subjects, surface-based statistical maps showing clusters of significant (p < 0.05) (See Figure 5).
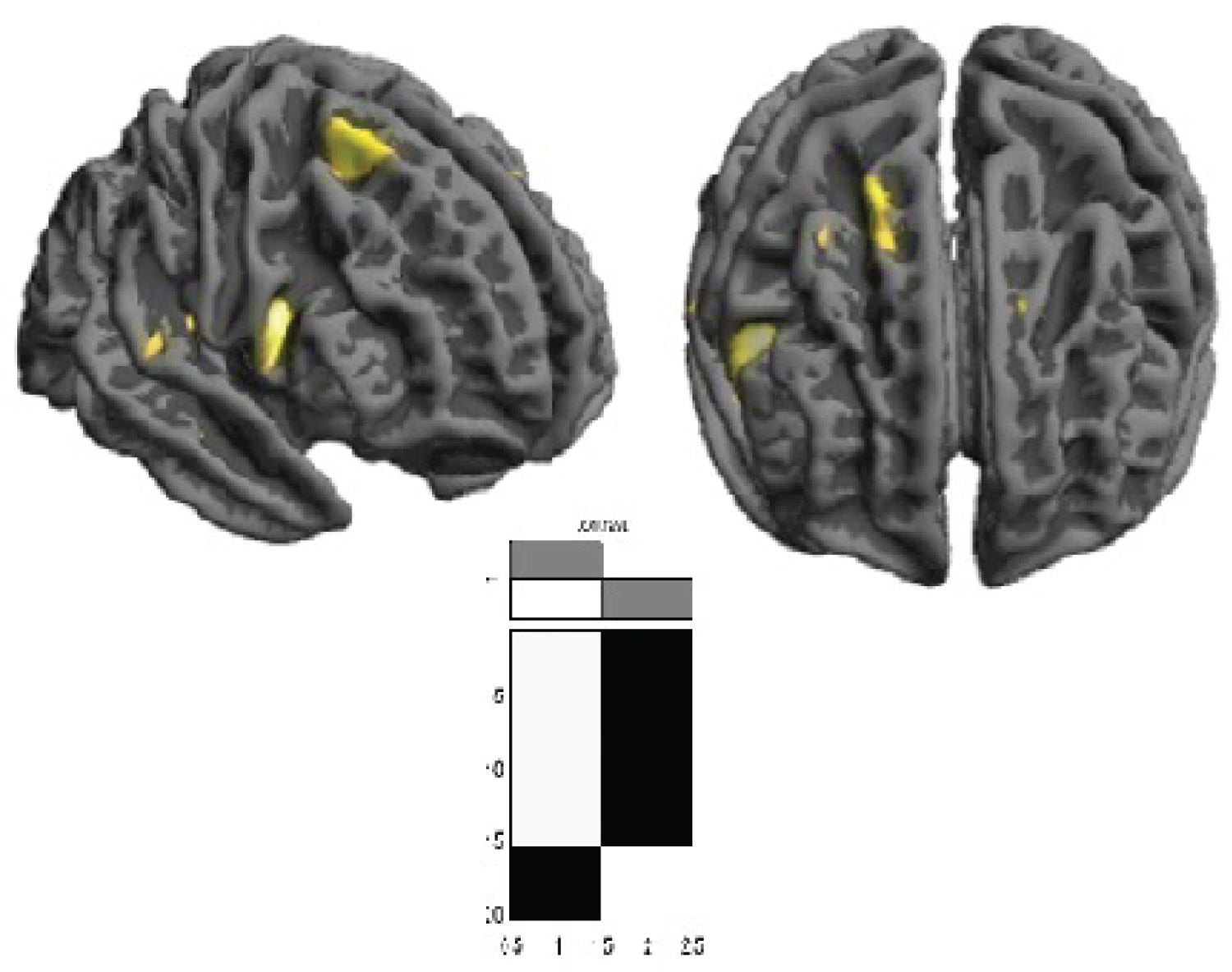 Figure 5: Cortical thickness group differences between ALS vs. healthy subjects. Surface-based statistical maps showing clusters of significant cortical thinning in ALS patients. (p < 0.05)
View Figure 5
Figure 5: Cortical thickness group differences between ALS vs. healthy subjects. Surface-based statistical maps showing clusters of significant cortical thinning in ALS patients. (p < 0.05)
View Figure 5
Regression analysis
Analysis of cortical thickness in relation with disease duration
It showed that mean cortical thickness diminishes when disease duration increasing (p = 0.00) See Table 5a.
Table 5a: Regression summary for dependent variable: Mean cortical thickness over the entire brain surface on ALS patients in relation with: (a) disease duration. View Table 5a
Analysis of cortical thickness in relation with ALSFRS score
It showed that mean cortical thickness diminishes when ALSFRS score is worse (p = 0.00) See Table 5b.
Table 5b: Regression summary for dependent variable: Mean cortical thickness over the entire brain surface on ALS patients in relation with: (b) ALSFRS-R score. View Table 5b
Great number of studies have reported abnormalities of brain of ALS patients. Some of these have reported abnormalities on cortex and deep nuclei, others have showed abnormalities on different motor and not motor tract, including sensory tracts, which has revolutionized the concept of ALS is a pure motor disease. Nevertheless, some of these abnormalities are not related with any clinical sign. This particular aspect remains unclear.
Stämpfli in 2018 demonstrated a significant deterioration of the white matter integrity in ALS patients, as reflected by reduced fibre density (FD) or fibre number and mean diffusion signal (MDS) values. Significant decreases of both parameters were found along CST and thalamic radiation, body of the corpus callosum as well as the forceps, uncinated fasciculus, superior longitudinal fasciculus, inferior longitudinal fasciculus, and inferior fronto-occipital fasciculus [11].
Geraldo in 2018 demonstrated that MD and AD maps showed significant differences between ALS patients and healthy controls in the CST and in the prefrontal white matter in the right cerebral hemisphere (p < 0.05). They also found increased of MD, AD, and RD in extra-motor frontal areas in ALS patients relative to controls [12].
Verber in 2019 demonstrated that MD elevation has to be considered as a marker in ALS diagnosis [13].
In ALS, FA reduction in the corticospinal tracts and corpus callosum is a consistent finding [14], which correlates with clinical measures of disease progression [15]. Nevertheless in our cases it was not significate in any of the evaluated tracts.
Bao in 2018 showed significate increased RD in premotor, primary motor, primary and secondary somatosensory cortical regions, corticospinal tract, superior corona radiata, posterior limb of internal capsule and cerebral peduncle; body of corpus callosum and part of bilateral external capsule. They found significate correlations between ALSFRS-R scores and the values of RD indicating that this DTI index might serve as imaging biomarker in evaluating the disease severity of ALS, and that the CST could be a reliable region for clinical monitoring [16]. Diagnostic sensitivity and specificity were 68 and 73%, respectively [17-19]. Previous studies have consistently demonstrated significant changes of the CST in ALS patients compared with healthy volunteers [20-29].
According to Pallebage-Gamarallage in 2018: DTI showed significant degree of white matter damage to the uncinated fasciculus, inferior and superior longitudinal fasciculus, arcuate fascicle, genu of corpus callosum, forceps minor and cingulum bundle [28]. For this reason, we decided add another non-motor tract like arcuate fascicle and optic radiation in our analysis.
In post-mortem studies, through the structural MRI, some authors showed lateral corticospinal tract hyperintensity along the scanned segment of the spinal cord, quantitative evaluation of the same region on the corresponding MRI plane demonstrated significant decrease in average FA value (p < 0.01) and significant increase in average MD values (p < 0.01) in comparison to the normal appearing white matter region [27,28].
Other post-mortem studies have demonstrated absence of Betz cells from Layer V of the precentral gyrus in patients with ALS and that the remaining pyramidal cells were significantly smaller than those seen in healthy controls [29,30].
Stämpfli showed significant changes in the fibre density values with disease progression. The fibre density values were decreased in parts of the CST, the thalamic radiation, the body of the corpus callosum, arcuate and uncinated fasciculus and in various association fibers [11]. Our results are according with Stämpfli´s results.
Stämpfli explained that fibre density may be more sensitive to white matter changes than the FA value and may permit evaluating pathological processes at an earlier stage of the disease [11].
In our opinion number of tracts or fibre density is a robust parameter to evaluate neurodegenerative diseases, at the beginning and in the diseases progress.
In relation to correlation with clinic abnormalities, Bao showed there was a negative correlation between the mean RD values and ALSFRS-R scores (r = -0.439, p = 0.011) on the left CST of ALS patients [16].
Stämpfli´s analysis did not reveal significant correlations of the diffusion parameters with disease severity as measured by the ALSFRS-R [11].
Sarsilmaz in 2018 demonstrated that there was a significant correlation between FA abnormalities with symptoms/disease duration and upper and lower motor neuron scores (p < 0.05). Statistical analysis indicated a strong correlation with El Escorial criteria, r-ALSFRS, and disease duration (p < 0.01) [31].
Qiu in 2019 found focal grey matter atrophy in the left precentral gyrus in patients with ALS in VBM analysis [29].
Steinbach in 2020 showed that ALS patients had significantly decreased of GM density in the medial, inferior-frontal, and temporal lobes; in the parietal, occipital and cerebellar and sub-cortical regions (p < 0.01). WM density was decreased in patients primarily in the bilateral frontal and parietal regions, extending down to the brainstem; in the bilateral temporal lobes, but not within cerebellar projections (p < 0.01) [32].
Trojsi in 2020 demonstrated GM atrophy (p < 0.05) in left precentral gyri, left frontal pole, right supramarginal gyrus, right inferior temporal gyrus and putamen [33].
In recent years imaging in ALS confirmed extensive extra-motor pathology in cerebellar, extrapyramidal, subcortical, hippocampal hypothalamic, brainstem, and frontotemporal involvement. Compared to HCs, patients with ALS showed at baseline regional [34-40].
Our results are according with other author´s results. And we consider future connectivity studies will clarify the significance of non-motor structures abnormalities.
Nijboer and previously other authors reported posterior cortical atrophy in some phenotype of ALS patients, thought conventional and post-processing MRI images. This pathological finding has been observed in Alzheimer disease too [41-44]. In our patients, posterior areas were very affected, it was demonstrated by different techniques.
Wirth in 2018 had done a cortical thickness analysis focused on precentral and postcentral regions of interest. They showed cortical thickness was not significantly different between ALS patients and healthy controls (p = 0.258) [45].
Meyer and other authors have done post-mortem and MRI studies and they have showed approximately 1.5 times greater cortical thickness of the precentral compared to the postcentral cortex in ALS patients [46-48].
Meadowcroft in 2015 observed cortical thinning in ALS patients with faster progression or advanced stage of disease [49].
Kropf and another authors have demonstrated that the significant cortical thinning of the postcentral gyrus occurring in ALS, and it is correlated with disease severity [50,51].
Abidi in 2019 described the right paracentral gyrus exhibited volumetric (p = 0.05) and thickness (p = 0.049) reduction in the upper motor neuron predominant cohort compared to the lower motor neuron predominant group [52].
Wirth in 2018 showed that ALSFRS-R sum scores correlated with cortical thickness of the precentral ventral cortex (p = 0.009) and the postcentral ventral region (p = 0.032) [45].
In our analysis different brain structures were observed affected in ALS patients:
Grey matter:
Motor structures: Frontal and parietal lobes, cerebellum, medulla oblongata, tectum mesencephalic, claustrum, globe pallidum, putamen and amygdalin nucleus.
Non-motor structures: Occipital lobe, cingulate gyrus, paracentral lobe, precuneus, uncus, gyrus parahipocampallis, gyrus lingual, insula, thalamic nucleus.
White matter:
Motor structures: Corticospinal tract at midbrain, pons and medulla oblongata, internal capsule, white matter of frontal and parietal areas.
Non-motor structures: corpus callosum, external capsule, optical radiation, lower and middle longitudinal fascicle, white matter of occipital areas.
Axonal degeneration seems to be the primary abnormality of main tracts, they show diminish of fibre numbers, diameter, RD and increase of MD. The tracts appear more affected when disease duration increase and when ALSFRS score gets worse.
Cortical thickness was diminished in ALS patients on pre-central gyrus, parietal posterior areas and postero-superior part of frontal lobe. It is more affected with the disease duration increase and when ALSFRS score gets worse.
In our ALS patient’s series was demonstrated morphologic abnormalities of grey and white mater of cortex, subcortical structures, cerebellum and brainstem. These abnormalities increase when clinical aspects get worse.
The authors report no conflicts of interest.
The author reports there are no competing interests to declare.