Department of Radiation Oncology, University of Health Sciences, Gulhane Medical Faculty, Ankara, Turkey
Abstract
Meningiomas are the most common intracranial benign tumors. While an indolent natural history is typical for benign meningiomas, a wide spectrum of symptoms may occur depending on lesion location and proximity to critical neurovascular structures. A multidisciplinary approach may be warranted for optimal management of meningiomas. In the scarcity of prospective, randomized controlled studies to dictate treatment algorithms, decision making for treatment is based primarily on mostly retrospective data. Surgery plays a central role for meningioma management, however, lesions located at eloquent brain regions in vicinity of critical neurovascular structures may not be amenable to complete surgical removal due to excessive risk of complications. In this context, radiation therapy offers a viable complementary or definitive treatment modality for management of selected patients with meningiomas. Radiosurgery has emerged as a sophisticated form of radiation therapy allowing precisely focused radiation beams to be delivered to well-defined targets under stereotactic localization and image guidance. There is growing body of evidence substantiating the role of radiosurgery in the form of stereotactic radiosurgery (SRS) or fractionated stereotactic radiotherapy (FSRT) for management of meningiomas. Herein, we review the utility of radiosurgery for meningiomas in light of the literature.
Keywords
Meningioma, Radiosurgery, Stereotactic radiosurgery (SRS), Fractionated stereotactic radiotherapy (FSRT)
Introduction
Meningiomas are the most common intracranial benign tumors accounting for approximately one third of all diagnosed intracranial neoplasms [1-9]. These dural-based tumors are thought to originate from arachnoid cap or meningothelial cells which are present in the arachnoid layer of the meninges [10,11]. Age of presentation is mostly in the 6th to 8th decades of life with the incidence increasing with age [4,5]. Histologically, meningiomas are classified as benign, atypical, or anaplastic (malignant) as per the World Health Organization (WHO) classification scheme with WHO grade I benign meningiomas being the most common type [5,6,11]. According to WHO classification, histological subtypes of WHO grade I meningioma include meningothelial (syncytial) meningioma, psammomatous meningioma, fibroblastic (fibrous) meningioma, transitional (mixed) meningioma, angiomatous (vascular) meningioma, secretory meningioma, lymphoplasmacyte-rich meningioma, metaplastic meningioma, and microcystic meningioma [12]. Histological subtypes of WHO grade II meningioma include chordoid meningioma, clear cell meningioma, and atypical meningioma [12]. Histological subtypes of WHO grade III meningioma include anaplastic (malignant) meningioma, papillary meningioma, and rhabdoid meningioma [12]. Most commonly seen histopathological types in decreasing order include meningothelial meningiomas, fibroblastic meningiomas, transitional meningiomas, psammomatous meningiomas, and other types [12].
Meningiomas may occur in various locations within the central nervous system (CNS). Most common location is the supratentorial region, followed by the base of skull and posterior fossa [12]. Meningiomas located at the supratentorial region include parasagittal meningiomas, convexity meningiomas, parafalcine meningiomas, and intraventricular meningiomas. Meningiomas located at the base of skull include sphonoid ridge meningiomas, olfactory groove meningiomas, tuberculum sellae meningiomas, petroclival meningiomas, intraorbital meningiomas, and cavernous sinus meningiomas. Meningiomas located at the posterior fossa include cerebellopontine angle meningiomas, cerebellar convexity meningiomas, foramen magnum meningiomas, peritorcular meningiomas, and jugular foramen meningiomas [12].
While benign meningiomas typically follow an indolent course as a slowly growing tumor, few patients with atypical or anaplastic meningiomas may suffer from invasive and recurrent disease occasionally accompanied by distant metastases [13-21].
Magnetic resonance imaging (MRI) is the principal imaging modality for meningiomas. However, imaging with computed tomography (CT) may help detecting tumoral calcifications, hyperostosis of the neighboring bone, and intraosseous growth of the tumor particularly for base of skull lesions [22]. MRI is useful for identification of the dural tail, if present, as post-contrast linear thickening of duramater adjacent to the meningioma lesion and offers improved contrast differentiation, and may allow for differentiating between intraaxial and extraaxial meningioma lesions. Typical appearance of meningioma is an exraaxial mass with well-defined borders. Homogeneous contrast enhancement is usual, however, areas of calcification or central necrosis may not enhance.
While incidental detection of meningiomas is not uncommon, affected patients may present with various symptoms depending on lesion location. Observation with vigilant neuroimaging may be an option for incidentally discovered and asymptomatic meningiomas [22-24]. Nevertheless, surgery plays a major role in management particularly when patients suffer from symptoms related to mass effect from large meningiomas located at surgically accessible locations. From the neurosurgical standpoint, Simpson described 5 grades of meningioma removal in 1957 and reported an association between aggressiveness of meningioma resection and subsequent tumor recurrences [25,26]. Despite considerable advances in neurosurgery, complete surgical removal of meningiomas may not be achievable in some cases particularly when tumors are seated at critical locations in intricate association with vital neurovascular structures [27-33]. While surgery remains to be a principal management modality for meningiomas, tumor recurrences are not uncommon even after complete surgical resection and cognitive or emotional problems including anxiety or depressive symptoms may develop after resection [23,32-36]. In this context, a multimodality management approach including less agressive surgery followed by adjuvant irradiation has been suggested as a viable therapeutic strategy for patients suffering from symptoms of mass effect in an attempt to improve the toxicity profile of treatment [28,37-41].
Stereotactic irradiation in the form of Stereotactic Radiosurgery (SRS), Fractionated Stereotactic Radiotherapy (FSRT), or Stereotactic Body Radiation Therapy (SBRT) has emerged as a viable therapeutic option for various intracranial and extracranial malign and benign conditions with encouraging treatment outcomes [42-59]. In the context of meningiomas, there has been accumulating experience on the utility of SRS and FSRT as a complementary or primary management for meningiomas [17,26,44,54,60-88].
Rationale for SRS
Enhanced killing of malignant cells may be achieved by high-precision radiosurgery techniques which preclude active tumor repopulation with delivery of limited treatment fractions of high fractional doses, providing effective tumoricidal activity through DNA injury along with induction of apoptosis and vascular endothelial damage [89-93]. Several converging, highly focused radiation beams are used for accurate targeting of intracranial lesions with SRS under excellent patient immobilization and image guidance. Multiple converging beams result in a tumoricidal dose of radiation at the target whilst sparing critical surrounding structures with steep dose gradients. Effectivity of high fraction doses may not be explained solely by the classical 4 Rs of radiotherapy which are repair, repopulation, redistribution and reoxygenation. Mechanism of tumoricidal activity include DNA injury along with induction of apoptosis and vascular endothelial damage which may be accompanied by other contributing factors currently under active investigation [93,94]. Meningiomas may be regarded as late responding tissue thus requiring high biologically effective doses for effective radiotherapy, which render SRS an appealing treatment modality. Typically higher biologically doses achieved with radiosurgery compared to conventionally fractionated radiotherapy may result in higher rates of tumor control with excellent critical organ sparing through steep dose gradients around the target. From the standpoint of patient convenience, radiosurgery may confer improved patient compliance with its typically condensed radiation treatment schedule achieving shortening of overall radiotherapy course and recovery as an outpatient procedure. With widespread adoption of innovative technologies improving treatment precision with decreased costs, radiosurgery started to serve as an affordable and viable radiotherapeutic strategy comprising an indespensable part of both neurosurgery and radiation oncology practice [95-97].
Decision making for treatment of meningiomas with radiosurgery should be made after multidisciplinary evaluation of patients based on lesion size, lesion location and proximity to critical neurovascular structures, symptomatology, patients' comorbidities, patient preferences, treatment availability and accessibility. Radiosurgery in the form of SRS may be judiciously used for management of well-circumscribed, small meningioma lesions with a diameter of < 3 cm and volume of < 10-15 cc located at a ≥ 2-3 mm distance away from the optic apparatus while FSRT may offer a viable radiosurgical option with an improved toxicity profile for management of larger meningioma lesions located at critical locations [98-100]. Considering the dose constraint of 8-10 Gy for the optic apparatus in the setting of single-fraction radiosurgery, FSRT may be well-suited for treatment of meningioma lesions abutting or in the vicinity of the optic apparatus. The rationale behind FSRT is to exploit the advantage of fractionation by means of repair and repopulation of normal tissues with potential for improved critical organ sparing whilst maintaining high-precision radiation delivery through excellent patient immobilization under image guidance with contemporary radiosurgical techniques.
Outcomes of Radiosurgery for WHO Grade I Benign Meningioma
Overall, studies of radiosurgery in the form of SRS and FSRT for meningioma consistently suggest improved local control rates both in the primary and salvage treatment setting [17,26,44,54,60-88,96-100,101-105] (Figure 1a and Figure 1b).
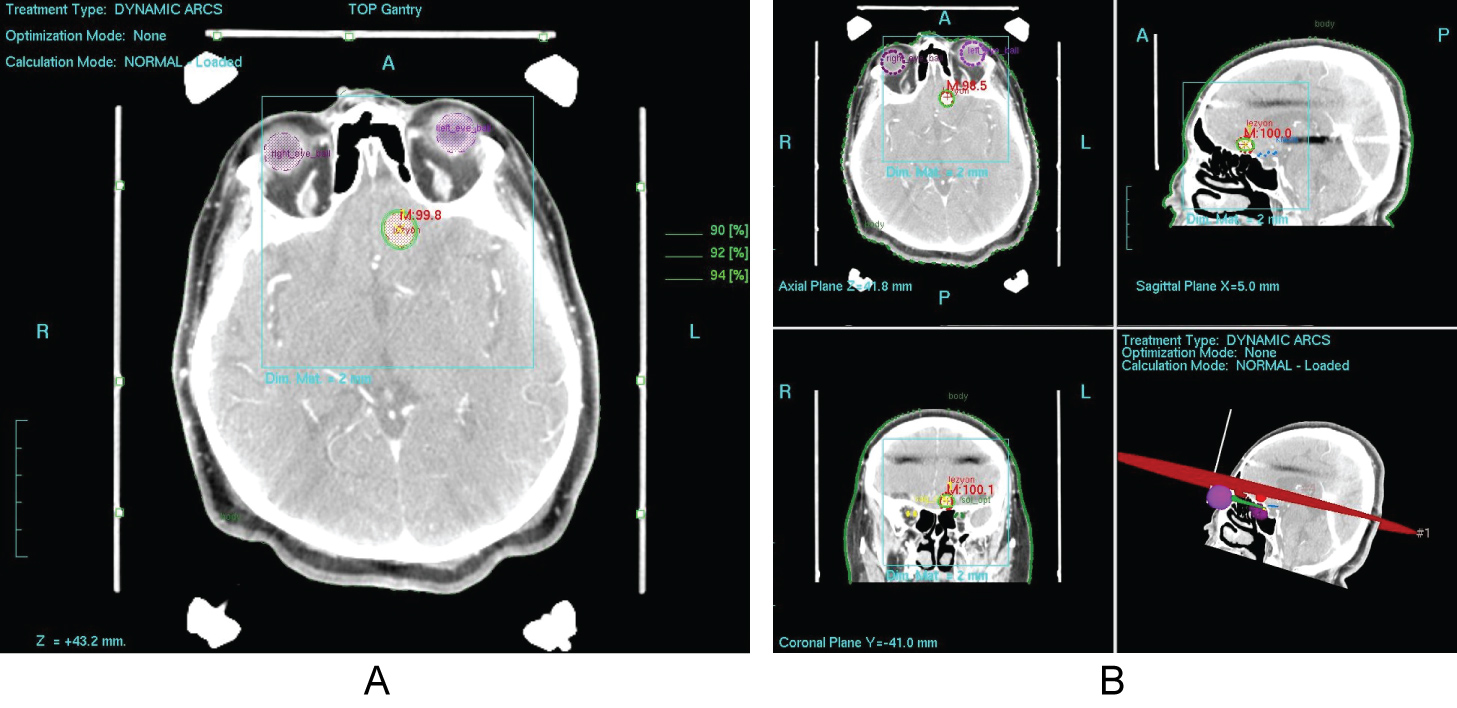 Figure 1: a,b) Treatment planning images of a patient with meningioma treated using SRS at our department.
View Figure 1
Figure 1: a,b) Treatment planning images of a patient with meningioma treated using SRS at our department.
View Figure 1
Treatment planning for radiosurgery warrants precise target localization. To achieve this goal, imaging with both CT and MR is used for radiosurgery treatment planning. Fusion of CT and MRI allows for improved definition of target and critical organs. Target volume for single fraction radiosurgery typically consists of the contrast enhancing lesion on MRI while an additional margin of 1-2 mm may be used for FSRT under stereotactic immobilization and image guidance.
Critical organs may include the brainstem, optic apparatus, cochlea, and other eloquent brain regions depending on location of the meningioma lesion. While establishing strict dose constraints for radiosurgery may be considered challenging due to the rapid dose fall-off and relatively small irradiated volumes, optic apparatus and brainstem doses below the range of 8-12 Gy may be preferred in single fraction radiosurgery treatments to minimize the risk of radiation induced complications [106,107].
Several factors including the lesion size and volume, proximity to surrounding critical organs and details of prior radiotherapy treatments should be considered in determination of the prescription dose for radiosurgery, however, single fraction doses in the range of 10 to 18 Gy have been typically used for SRS of benign meningiomas [101]. A minimum peripheral tumor dose of ≤ 10 Gy has been associated with higher risk of failure when compared to a dose of ≥ 12 Gy in a study by Ganz, et al. using Gamma Knife radiosurgery for meningiomas [102].
In a study of 188 patients with benign or presumed benign meningioma treated with surgery or SRS alone, Pollock, et al. reported equivalent 7-year progression free survival (PFS) of 95% and 96% for SRS and Simpson grade I surgery, respectively [97]. Nevertheless, tumor control was better with SRS in the setting of less extensive surgery, and SRS was suggested as the primary management modality for meningiomas not amenable to Simpson grade I surgery [97].
In a study of 972 patients with 1045 intracranial meningiomas treated during a 18-year period, Kondziolka, et al. reported an overall control rate of 93% and a 10-year local tumor control rate of 91% for benign meningiomas [103]. Actuarial rates of tumor control was 97%, 87.2%, and 87.2% at 5, 10, and 15 years, respectively [103].
Kessel, et al. recently reported treatment outcomes of patients with meningiomas receiving high-precision radiotherapy as SRS, FSRT, or intensity-modulated radiation therapy [104]. At a median follow-up of 7.2 years, overall survival (OS) rate for the 147 patients with low grade meningiomas was 97%, 85%, and 64% at 3, 10, and 15 years, respectively [104]. Local control rate was 91%, 87%, and 86% at 3, 5, and 10 years, respectively. Long term toxicity was low with excellent local control [104].
A systematic review and meta analysis by Pinzi, et al. reported an estimated 5-year disease control rate of 87% to 100% and a 10-year disease control rate of 67% to 100% at 10 years with SRS for WHO grade I meningiomas [105]. Progression free survival (PFS) rates at 3, 5, and 10 years were 91.3% to 100%, 78% to 98.9%, and 53.1% to 97.2%, respectively [105]. Overall syptom control and toxicity rates were 92.3% and 8.1%, respectively [105].
Outcomes of Radiosurgery for WHO Grade II-III Meningiomas
Although studies of radiosurgery typically focused on management of WHO grade I benign meningiomas, radiosurgery has also been utilized for WHO grade II and III meningiomas [103,105,104,108-113].
Due to an increased risk of local recurrence, higher prescription doses than single fraction radiosurgery doses may be required for radiosurgical management of WHO grade II-III meningiomas. A study by Sethi, et al. assessing dose-response relationships for meningioma radiosurgery revealed that treatment dose had a significant effect on local control of meningiomas [103]. Out of the total 108 meningioma lesions treated with Gamma Knife radiosurgery, 20 lesions were WHO grade II-III meningiomas and radiosurgery dose was found to be the strongest determinant of local failure for high grade meningiomas. Authors suggested dose escalation beyond 16 Gy to improve local control of WHO grade II-III meningiomas [103].
In this context, given the probability of differential responses to radiosurgery for meningiomas with different WHO grades, delivery of higher radiosurgery doses may be considered for achieving improved local control for WHO grade II-III meningiomas [103,114].
In the study by Kondziolka, et al. evaluating radiosurgery as definitive treatment modality for meningioma management, tumor control rate was 50% and 17% for WHO grade II and III meningiomas, respectively [105].
The study by Kessel, et al. assessing long-term results of high-precision radiotherapy for meningiomas including 43 high-grade meningioma patients with 40 WHO grade II and 3 WHO grade III meningiomas, local control rate was 67% and 55% at 3 and 5 years, respectively for patients with atypical meningioma while OS rate was 91%, 62%, and 50% at 3, 10, and 15 years, respectively [106].
In the study by Aboukais, et al. assessing the utility of radiosurgery for WHO grade II meningiomas, 27 patients were treated using a radiosurgery dose in the range of 12 to 21 Gy (mean 15.2 Gy) [111]. At a mean follow-up of 56.4 months, actuarial local control rates for all patients were 75%, 52%, and 40% at 1, 2 and 3 years, respectively. The authors concluded that radiosurgery may be considered for management of WHO grade II meningiomas [111].
Choi, et al. evaluated Cyberknife SRS for management of 25 patents with WHO grade II meningiomas [112]. Using a median marginal dose of 22 Gy (range: 16-30 Gy) delivered in 1 to 4 fractions (median 1 fraction), actuarial local control rates for all patients were 94%, 94%, and 74% at 1, 2, and 3 years, respectively [112].
In a comprehensive literature review, Ding, et al., assessed the utility of radiosurgery for WHO grade II-III meningiomas based on 19 radiosurgery series [113]. Median margin dose was 14-21 Gy. Typical margin dose was 16-20 Gy and 18-20 Gy for WHO grade II and WHO grade III meningiomas, respectively. Progression free survival (PFS) was in the range of 25% to 83% (median 59%) for WHO grade II tumors and 0% to 72% (median 13%) for WHO grade III tumors. Median complication rate after radiosurgery was 8% [113].
Overall, accumulating data suggest a potential role for radiosurgery in the management of WHO grade II-III meningiomas despite the need for further supporting evidence.
Conclusion and Future Directions
Meningiomas are the most common intracranial benign tumors accounting for approximately one third of all diagnosed intracranial neoplasms. Although the majority of meningiomas are WHO Grade I with a typically indolent natural history, they may cause significant patient suffering depending on their location. The literature is short of prospective, randomized controlled studies to dictate treatment algorithms. In this context, decision making for treatment is based primarily on mostly retrospective data. While surgery remains to be a major therapeutic modality, radiosurgery in the form of SRS and FSRT has emerged as a viable treatment strategy for meningioma management. In this context, there is growing body of evidence supporting the role of radiosurgery as a complementary or definitive treatment modality for management of meningiomas. Lesions located at eloquent brain regions in vicinity of critical neurovascular structures may be judiciously treated using radiosurgery to avoid the risks of surgical complications. In some cases, it may be feasible to utilize a combined modality approach including debulking surgery to relieve the mass effect followed by radiosurgery to improve local disease control. Molecular characterization and gene expressing for meningiomas is currently under active investigation to shed light on potential role of systemic therapies for management.
In conclusion, radiosurgery offers a viable and non-invasive therapeutic strategy for management of meningiomas. Ever-increasing improvements in technology and advances in neuroimaging, radiosurgery techniques, equipment, treatment planning and delivery systems has rendered radiosurgery an attractive treatment modality with growing utilization.
References
-
Hashiba T, Hashimoto N, Maruno M, Izumoto S, Suzuki T, et al. (2006) Scoring radiologic characteristics to predict proliferative potential in meningiomas. Brain Tumor Pathol 23: 49-54.
-
Surawicz TS, McCarthy BJ, Kupelian V, Jukich PJ, Bruner JM, et al. (1999) Descriptive epidemiology of primary brain and CNS tumors: Results from the central brain tumor registry of the United States, 1990-1994. Neuro Oncol 1: 14-25.
-
Staneczek W, Jänisch W (1992) Epidemiologic data on meningiomas in East Germany 1961-1986: incidence, localization, age and sex distribution. Clin Neuropathol 11: 135-141.
-
Bondy M, Ligon BL (1996) Epidemiology and etiology of intracranial meningiomas: A review. J Neurooncol 29: 197-205.
-
Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, et al. (2005) Epidemiology of intracranial meningioma. Neurosurgery 57: 1088-1095.
-
Deltour I, Johansen C, Auvinen A, Feychting M, Klaeboe L, et al. (2009) Time trends in brain tumor incidence rates in Denmark, Finland, Norway, and Sweden, 1974-2003. J Natl Cancer Inst 101: 1721-1724.
-
Gigineishvili D, Gigineishvili T, Tsiskaridze A, Shakarishvili R (2014) Incidence rates of the primary brain tumours in Georgia-a population-based study. BMC Neurol 14: 29.
-
Dolecek TA, Dressler EV, Thakkar JP, Liu M, Al-Qaisi A, et al. (2015) Epidemiology of meningiomas post-Public Law 107-206: The benign brain tumor cancer registries amendment act. Cancer 121: 2400-2410.
-
Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, et al. (2016) CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosedin the United States in 2009-2013. Neuro Oncol 18: 1-75.
-
Pieper DR, Al-Mefty O, Hanada Y, Buechner D (1999) Hyperostosis associated with meningioma of the cranial base: Secondary changes or tumor invasion. Neurosurgery 44: 742-746.
-
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, et al. (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114: 97-109.
-
Bhat AR, Wani MA, Kirmani AR, Ramzan AU (2014) Histological-subtypes and anatomical location correlated in meningeal brain tumors (meningiomas). J Neurosci Rural Pract 5: 244-249.
-
Buerki RA, Horbinski CM, Kruser T, Horowitz PM, James CD, et al. (2018) An overview of meningiomas. Future Oncol 14: 2161-2177.
-
Dutta SW, Peterson JL, Vallow LA, Mahajan A, Rosenfeld SS, et al. (2018) National care among patients with WHO grade I intracranial meningioma. J Clin Neurosci 55: 17-24.
-
Shakir SI, Souhami L, Petrecca K, Mansure JJ, Singh K, et al. (2018) Prognostic factors for progression in atypical meningioma. J Neurosurg Jan 19: 1-9.
-
Liu X, Shan B, Wang M, Xu J (2018) World Health Organization Grade II meningiomas: The role of adjuvant/salvage gamma knife surgery after initial surgery and prognostic factor assessment. World Neurosurg 109: e352-e362.
-
Cohen-Inbar O, Lee CC, Sheehan JP (2016) The contemporary role of stereotactic radiosurgery in the treatment of meningiomas. Neurosurg Clin N Am 27: 215-228.
-
Rogers L, Barani I, Chamberlain M, Kaley TJ, McDermott M, et al. (2015) Meningiomas: Knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg 122: 4-23.
-
Kaur G, Sayegh ET, Larson A, Bloch O, Madden M, et al. (2014) Adjuvant radiotherapy for atypical and malignant meningiomas: A systematic review. Neuro Oncol 16: 628-636.
-
Chen CM, Huang AP, Kuo LT, Tu YK (2011) Contemporary surgical outcome for skull base meningiomas. Neurosurg Rev 34: 281-296.
-
Mawrin C, Perry A (2010) Pathological classification and molecular genetics of meningiomas. J Neurooncol 99: 379-391.
-
Goldbrunner R, Minniti G, Preusser M, Jenkinson MD, Sallabanda K, et al. (2016) EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol 17: e383-e391.
-
Sughrue ME, Kane AJ, Shangari G, Rutkowski MJ, McDermott MW, et al. (2010) The relevance of simpson grade I and II resection in modern neurosurgical treatment of world health organization grade I meningiomas. J Neurosurg 113: 1029-1035.
-
Vernooij MW, Ikram MA, Tanghe HL, Vincent AJ, Hofman A, et al. (2007) Incidental findings on brain MRI in the general population. N Engl J Med 357: 1821-1828.
-
Simpson D (1957) The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 20: 22-39.
-
Pollock BE, Stafford SL, Link MJ (2013) Stereotactic radiosurgery of intracranial meningiomas. Neurosurg Clin N Am 24: 499-507.
-
Van Havenbergh T, Carvalho G, Tatagiba M, Plets C, Samii M (2003) Natural history of petroclival meningiomas. Neurosurgery 52: 55-62.
-
Dufour H, Muracciole X, Métellus P, Régis J, Chinot O, et al. (2001) Long-term tumor control and functional outcome in patients with cavernous sinus meningiomas treated by radiotherapy with or without previous surgery: Is there an alternative to aggressive tumor removal? Neurosurgery 48: 285-296.
-
Sen C, Hague K (1997) Meningiomas involving the cavernous sinus: Histological factors affecting the degree of resection. J Neurosurg 87: 535-543.
-
Larson JJ, van Loveren HR, Balko G, Tew JM Jr. (1995) Evidence of meningioma infiltration into cranial nerves: Clinical implications for cavernous sinus meningiomas. J Neurosurg 83: 596-599.
-
Kotapka MJ, Kalia KK, Martinez AJ, Sekhar LN (1994) Infiltration of the carotid artery by cavernous sinus meningioma. J Neurosurg 81: 252-255.
-
DeMonte F, Smith HK, al-Mefty O (1994) Outcome of aggressive removal of cavernous sinus meningiomas. J Neurosurg 81: 245-251.
-
Mirimanoff RO, Dosoretz DE, Linggood RM, Ojemann RG, Martuza RL (1985) Meningioma: Analysis of recurrence and progression following neurosurgical resection. J Neurosurg 62: 18-24.
-
Mathiesen T, Lindquist C, Kihlström L, Karlsson B (1996) Recurrence of cranial base meningiomas. Neurosurgery 39: 2-7.
-
Stafford SL, Perry A, Suman VJ, Meyer FB, Scheithauer BW (1998) Primarily resected meningiomas: Outcome and prognostic factors in 581 Mayo Clinic patients, 1978 through 1988. Mayo Clin Proc 73: 936-942.
-
van der Vossen S, Schepers VP, Berkelbach van der Sprenkel JW, Visser-Meily JM, Post MW (2014) Cognitive and emotional problems in patients after cerebral meningioma surgery. J Rehabil Med 46: 430-437.
-
Talacchi A, Muggiolu F, De Carlo A, Nicolato A, Locatelli F, et al. (2016) Recurrent atypical meningiomas: Combining surgery and radiosurgery in one effective multimodal treatment. World Neurosurg 87: 565-572.
-
Aboukais R, Zairi F, Reyns N, Le Rhun E, Touzet G, et al. (2014) Surgery followed by radiosurgery: A deliberate valuable strategy in the treatment of intracranial meningioma. Clin Neurol Neurosurg 124: 123-126.
-
Di Maio S, Ramanathan D, Garcia-Lopez R, Rocha MH, Guerrero FP, et al. (2012) Evolution and future of skull base surgery: The paradigm of skull base meningiomas. World Neurosurg 78: 260-275.
-
Couldwell WT, Kan P, Liu JK, Apfelbaum RI (2006) Decompression of cavernous sinus meningioma for preservation and improvement of cranial nerve function. Technical note. J Neurosurg 105: 148-152.
-
Abdel-Aziz KM, Froelich SC, Dagnew E, Jean W, Breneman JC, et al. (2004) Large sphenoid wing meningiomas involving the cavernous sinus: Conservative surgical strategies for better functional outcomes. Neurosurgery 54: 1375-1383.
-
Dincoglan F, Sager O, Demiral S, Uysal B, Gamsiz H, et al. (2017) Radiosurgery for recurrent glioblastoma: A review article. Neurol Disord Therap 1: 1-5.
-
Dincoglan F, Sager O, Gamsiz H, Uysal B, Demiral S, et al. (2012) Stereotactic radiosurgery for intracranial tumors: A single center experience. Gulhane Med J 54: 190-198.
-
Demiral S, Dincoglan F, Sager O, Gamsiz H, Uysal B, et al. (2016) Hypofractionated stereotactic radiotherapy (HFSRT) for who grade I anterior clinoid meningiomas (ACM). Jpn J Radiol 34: 730-737.
-
Dincoglan F, Beyzadeoglu M, Sager O, Demiral S, Gamsiz H, et al. (2015) Management of patients with recurrent glioblastoma using hypofractionated stereotactic radiotherapy. Tumori 101: 179-184.
-
Sager O, Dincoglan F, Beyzadeoglu M (2015) Stereotactic radiosurgery of glomus jugulare tumors: Current concepts, recent advances and future perspectives. CNS Oncol 4: 105-114.
-
Gamsiz H, Beyzadeoglu M, Sager O, Demiral S, Dincoglan F, et al. (2015) Evaluation of stereotactic body radiation therapy in the management of adrenal metastases from non-small cell lung cancer. Tumori 101: 98-103.
-
Dincoglan F, Sager O, Gamsiz H, Uysal B, Demiral S, et al. (2014) Management of patients with ≥4 brain metastases using stereotactic radiosurgery boost after whole brain irradiation. Tumori 100: 302-306.
-
Demiral S, Beyzadeoglu M, Sager O, Dincoglan F, Gamsiz H, et al. (2014) Evaluation of linear accelerator (linac)-based stereotactic radiosurgery (srs) for the treatment of craniopharyngiomas. UHOD 24: 123-129.
-
Sager O, Beyzadeoglu M, Dincoglan F, Gamsiz H, Demiral S, et al. (2014) Evaluation of linear accelerator-based stereotactic radiosurgery in the management of glomus jugulare tumors. Tumori 100: 184-188.
-
Gamsiz H, Beyzadeoglu M, Sager O, Dincoglan F, Demiral S, et al. (2014) Management of pulmonary oligometastases by stereotactic body radiotherapy. Tumori 100: 179-183.
-
Sager O, Beyzadeoglu M, Dincoglan F, Uysal B, Gamsiz H, et al. (2014) Evaluation of linear accelerator (LINAC)-based stereotactic radiosurgery (SRS) for cerebral cavernous malformations: A 15-year single-center experience. Ann Saudi Med 34: 54-58.
-
Sager O, Beyzadeoglu M, Dincoglan F, Demiral S, Uysal B, et al. (2013) Management of vestibular schwannomas with linear accelerator-based stereotactic radiosurgery: A single center experience. Tumori 99: 617-622.
-
Dincoglan F, Beyzadeoglu M, Sager O, Uysal B, Demiral S, et al. (2013) Evaluation of linear accelerator-based stereotactic radiosurgery in the management of meningiomas: A single center experience. J BUON 18: 717-722.
-
Demiral S, Beyzadeoglu M, Uysal B, Oysul K, Kahya YE, et al. (2013) Evaluation of stereotactic body radiotherapy (SBRT) boost in the management of endometrial cancer. Neoplasma 60: 322-327.
-
Surenkok S, Sager O, Dincoglan F, Gamsiz H, Demiral S, et al. (2012) Stereotactic radiosurgery in pituitary adenomas: A single center experience. UHOD - Uluslararasi Hematoloji-Onkoloji Dergisi 22: 255-260.
-
Dincoglan F, Sager O, Gamsiz H, Demiral S, Uysal B, et al. (2012) Management of arteriovenous malformations by stereotactic radiosurgery: A single center experience. UHOD - Uluslararasi Hematoloji-Onkoloji Dergisi 22: 107-112.
-
Dincoglan F, Beyzadeoglu M, Sager O, Oysul K, Sirin S, et al. (2012) Image-guided positioning in intracranial non-invasive stereotactic radiosurgery for the treatment of brain metastasis. Tumori 98: 630-635.
-
Sirin S, Oysul K, Surenkok S, Sager O, Dincoglan F, et al. (2011) Linear accelerator-based stereotactic radiosurgery in recurrent glioblastoma: a single center experience. Vojnosanit Pregl 68: 961-966.
-
Yazici O, Adas YG, Kekilli E, Calikoglu T, Altundag MB, et al. (2018) Intracranial meningioma: Experience with stereotactic radiotherapy. JBUON 23: 1169-1173.
-
Park KJ, Kano H, Iyer A, Liu X, Tonetti DA, et al. (2018) Gamma Knife stereotactic radiosurgery for cavernous sinus meningioma: Long-term follow-up in 200 patients. J Neurosurg 20: 1-10.
-
Hasegawa H, Hanakita S, Shin M, Koga T, Takahashi W, et al. (2018) Single-Fractionated stereotactic radiosurgery for ıntracranial meningioma in elderly patients: 25-year experience at a single ınstitution. Oper Neurosurg (Hagerstown) 14: 341-350.
-
Mehta GU, Zenonos G, Patibandla MR, Lin CJ, Wolf A, et al. (2018) Outcomes of stereotactic radiosurgery for foramen magnum meningiomas: an international multicenter study. J Neurosurg 129: 383-389.
-
Manabe Y, Murai T, Ogino H, Tamura T, Iwabuchi M, et al. (2017) Cyberknife stereotactic radiosurgery and hypofractionated stereotactic radiotherapy as first-line treatments for imaging-diagnosed intracranial meningiomas. Neurol Med Chir (Tokyo) 57: 627-633.
-
Cohen-Inbar O, Lee CC, Schlesinger D, Xu Z, Sheehan JP (2016) Long-Term results of stereotactic radiosurgery for skull base meningiomas. Neurosurgery 79: 58-68.
-
El-Khatib M, El Majdoub F, Hunsche S, Hoevels M, Kocher M, et al. (2015) Stereotactic LINAC radiosurgery for the treatment of typical intracranial meningiomas. Efficacy and safety after a follow-up of over 12 years. Strahlenther Onkol 191: 921-927.
-
Sheehan JP, Starke RM, Kano H, Barnett GH, Mathieu D, et al. (2015) Gamma Knife radiosurgery for posterior fossa meningiomas: a multicenter study. J Neurosurg 12: 1479-1489.
-
Hadelsberg U, Nissim U, Cohen ZR, Spiegelmann R (2015) LINAC radiosurgery in the management of parasagittal meningiomas. Stereotact Funct Neurosurg 93: 10-16.
-
Park SH, Kano H, Niranjan A, Monaco E 3rd, Flickinger JC, et al. (2015) Gamma Knife radiosurgery for meningiomas arising from the tentorium: A 22-year experience. J Neurooncol 121: 129-134.
-
Ding D, Starke RM, Kano H, Nakaji P, Barnett GH, et al. (2014) Gamma knife radiosurgery for cerebellopontine angle meningiomas: A multicenter study. Neurosurgery 75: 398-408.
-
Starke R, Kano H, Ding D, Nakaji P, Barnett GH, et al. (2014) Stereotactic radiosurgery of petroclival meningiomas: A multicenter study. J Neurooncol 119: 169-176.
-
Kondziolka D, Patel AD, Kano H, Flickinger JC, Lunsford LD (2016) Long-term outcomes after gamma knife radiosurgery for meningiomas. Am J Clin Oncol 39: 453-457.
-
Sheehan JP, Starke RM, Kano H, Kaufmann AM, Mathieu D, et al. (2014) Gamma knife radiosurgery for sellar and parasellar meningiomas: A multicenter study. J Neurosurg 120: 1268-1277.
-
Park SH, Kano H, Niranjan A, Flickinger JC, Lunsford LD (2014) Stereotactic radiosurgery for cerebellopontine angle meningiomas. J Neurosurg 120: 708-715.
-
Pollock BE, Stafford SL, Link MJ, Garces YI, Foote RL (2012) Single-fraction radiosurgery for presumed intracranial meningiomas: Efficacy and complications from a 22-year experience. Int J Radiat Oncol Biol Phys 83: 1414-1418.
-
dos Santos MA, de Salcedo JB, Gutiérrez Diaz JA, Calvo FA, Samblás J, et al. (2011) Long-term outcomes of stereotactic radiosurgery for treatment of cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys 81: 1436-1441.
-
Park HR, Lee JM, Park KW, Kim JH, Jeong SS, et al. (2018) Fractionated gamma knife radiosurgery as initial treatment for large skull base meningioma. Exp Neurobiol 27: 245-255.
-
Marchetti M, Bianchi S, Pinzi V, Tramacere I, Fumagalli ML, et al. (2016) Multisession radiosurgery for sellar and parasellar benign meningiomas: Long-term tumor growth control and visual outcome. Neurosurgery 78: 638-646.
-
Maranzano E, Draghini L, Casale M, Arcidiacono F, Anselmo P, et al. (2015) Long-term outcome of moderate hypofractionated stereotactic radiotherapy for meningiomas. Strahlenther Onkol 191: 953-960.
-
Kaul D, Budach V, Graaf L, Gollrad J, Badakhshi H (2015) Outcome of elderly patients with meningioma after image-guided stereotactic radiotherapy: A study of 100 cases. Biomed Res Int 2015: 868401.
-
Biau J, Khalil T, Verrelle P, Lemaire JJ (2018) Fractionated radiotherapy and radiosurgery of intracranial meningiomas. Neurochirurgie 64: 29-36.
-
Navarria P, Pessina F, Cozzi L, Clerici E, Villa E, et al. (2015) Hypofractionated stereotactic radiation therapy in skull base meningiomas. J Neurooncol 124: 283-289.
-
Fokas E, Henzel M, Surber G, Hamm K, Engenhart-Cabillic R (2014) Stereotactic radiotherapy of benign meningioma in the elderly: Clinical outcome and toxicity in 121 patients. Radiother Oncol 111: 457-462.
-
Fokas E, Henzel M, Surber G, Hamm K, Engenhart-Cabillic R (2014) Stereotactic radiation therapy for benign meningioma: Long-term outcome in 318 patients. Int J Radiat Oncol Biol Phys 89: 569-575.
-
Oermann EK, Bhandari R, Chen VJ, Lebec G, Gurka M, et al. (2013) Five fraction image-guided radiosurgery for primary and recurrent meningiomas. Front Oncol 3: 213.
-
Bria C, Wegner RE, Clump DA, Vargo JA, Mintz AH, et al. (2011) Fractionated stereotactic radiosurgery for the treatment of meningiomas. J Cancer Res Ther 7: 52-57.
-
Pacelli R, Cella L, Conson M, Tranfa F, Strianese D, et al. (2011) Fractionated stereotactic radiation therapy for orbital optic nerve sheath meningioma - a single institution experience and a short review of the literature. J Radiat Res 52: 82-87.
-
Onodera S, Aoyama H, Katoh N, Taguchi H, Yasuda K, et al. (2011) Long-term outcomes of fractionated stereotactic radiotherapy for intracranial skull base benign meningiomas in single institution. Jpn J Clin Oncol 41: 462-468.
-
Larson DA, Flickinger JC, Loeffler JS (1993) The radiobiology of radiosurgery. Int J Radiat Oncol Biol Phys 25: 557-561.
-
Song CW, Cho LC, Yuan J, Dusenbery KE, Griffin RJ, et al. (2013) Radiobiology of stereotactic body radiation therapy/stereotactic radiosurgery and the linear-quadratic model. Int J Radiat Oncol Biol Phys 87: 18-19.
-
Balagamwala EH, Chao ST, Suh JH (2012) Principles of radiobiology of stereotactic radiosurgery and clinical applications in the central nervous system. Technol Cancer Res Treat 11: 3-13.
-
Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, et al. (2003) Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 300: 1155-1159.
-
Brown JM, Carlson DJ, Brenner DJ (2014) The tumor radiobiology of SRS and SBRT: Are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys 88: 254-262.
-
Kirkpatrick JP, Meyer JJ, Marks LB (2008) The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol 18: 240-243.
-
Niranjan A, Madhavan R, Gerszten PC, Lunsford LD (2012) Intracranial radiosurgery: An effective and disruptive innovation in neurosurgery. Stereotact Funct Neurosurg 90: 1-7.
-
Bir SC, Patra DP, Maiti TK, Bollam P, Minagar A, et al. (2017) Direct comparison of gamma knife radiosurgery and microsurgery for small size meningiomas. World Neurosurg 101: 170-179.
-
Pollock BE, Stafford SL, Utter A, Giannini C, Schreiner SA (2003) Stereotactic radiosurgery provides equivalent tumor control to simpson grade 1 resection for patients with small-to medium-size meningiomas. Int J Radiat Oncol Biol Phys 55: 1000-1005.
-
Chung LK, Mathur I, Lagman C, Bui TT, Lee SJ, et al. (2017) Stereotactic radiosurgery versus fractionated stereotactic radiotherapy in benign meningioma. J Clin Neurosci 36: 1-5.
-
Kocher M, Treuer H, Hoevels M, Semrau R, Sturm V, et al. (2013) Endocrine and visual function after fractionated stereotactic radiotherapy of perioptic tumors. Strahlenther Onkol 189: 137-141.
-
Torres RC, Frighetto L, De Salles AA, Goss B, Medin P, et al. (2003) Radiosurgery and stereotactic radiotherapy for intracranial meningiomas. Neurosurg Focus 14: e5.
-
Sethi RA, Rush SC, Liu S, Sethi SA, Parker E, et al. (2015) Dose-response relationships for meningioma radiosurgery. Am J Clin Oncol 38: 600-604.
-
Ganz JC, Backlund EO, Thorsen FA (1993) The results of gamma knife surgery of meningiomas, related to size of tumor and dose. Stereotact Funct Neurosurg 61: 23-29.
-
Kondziolka D, Mathieu D, Lunsford LD, Martin JJ, Madhok R, et al. (2008) Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery 62: 53-58.
-
Kessel KA, Fischer H, Oechnser M, Zimmer C, Meyer B, et al. (2017) High-precision radiotherapy for meningiomas: Long-term results and patient-reported outcome (PRO). Strahlenther Onkol 193: 921-930.
-
Pinzi V, Biagioli E, Roberto A, Galli F, Rizzi M, et al. (2017) Radiosurgery for intracranial meningiomas: A systematic review and meta-analysis. Crit Rev Oncol Hematol 113: 122-134.
-
Mayo C, Martel MK, Marks LB, Flickinger J, Nam J, et al. (2010) Radiation dose-volume effects of optic nerves and chiasm. Int J Radiat Oncol Biol Phys 76: S28-S35.
-
Mayo C, Yorke E, Merchant TE (2010) Radiation associated brainstem injury. Int J Radiat Oncol Biol Phys 76: S36-S41.
-
Lagman C, Bhatt NS, Lee SJ, Bui TT, Chung LK, et al. (2017) Adjuvant radiosurgery versus serial surveillance following subtotal resection of atypical meningioma: A systematic analysis. World Neurosurg 98: 339-346.
-
Zhang M, Ho AL, D'Astous M, Pendharkar AV, Choi CY, et al. (2016) cyberknife stereotactic radiosurgery for atypical and malignant meningiomas.World Neurosurg 91: 574-581.
-
Wang WH, Lee CC, Yang HC, Liu KD, Wu HM, et al. (2016) Gamma knife radiosurgery for atypical and anaplastic meningiomas. World Neurosurg 87: 557-564.
-
Aboukais R, Zairi F, Lejeune JP, Le Rhun E, Vermandel M, et al. (2015) Grade 2 meningioma and radiosurgery. J Neurosurg 122: 1157-1162.
-
Choi CY, Soltys SG, Gibbs IC, Harsh GR, Jackson PS, et al. (2010) Cyberknife stereotactic radiosurgery for treatment of atypical (WHO grade II) cranial meningiomas. Neurosurgery 67: 1180-1188.
-
Ding D, Starke RM, Hantzmon J, Yen CP, Williams BJ, et al. (2013) The role of radiosurgery in the management of WHO Grade II and III intracranial meningiomas. Neurosurg Focus 35: E16.
-
Kawashima M, Suzuki SO, Ikezaki K, Matsushima T, Fukui M, et al. (2001) Different responses of benign and atypical meningiomas to gamma-knife radiosurgery: Report of two cases with immunohistochemical analysis. Brain Tumor Pathol 18: 61-66.
Citation
Demiral S, Dincoglan F, Sager O, Uysal B, Gamsiz H, et al. (2018) Contemporary Management of Meningiomas with Radiosurgery. Int J Radiol Imaging Technol 4:041. doi.org/10.23937/2572-3235.1510041
 Figure 1: a,b) Treatment planning images of a patient with meningioma treated using SRS at our department.
View Figure 1
Figure 1: a,b) Treatment planning images of a patient with meningioma treated using SRS at our department.
View Figure 1