Despite improvements in the morphological quality of lung images in recent years, using ultra-short echo time sequences [1], the low signal-to-noise ratio caused by the low proton density of lung tissue (~0.1 g/ml) and cardiac and respiratory motions that cause artifacts have been the cause of limitations in magnetic resonance imaging (MRI) [2]. Spatial resolution and diagnostic quality are then reduced. This is mainly evident in emergency situations of pulmonary embolism or in chronic bronchopulmonary diseases. To improve the BOLD signal, this study proposes a method using artificial intelligence techniques, particularly artificial neural networks, as a tool for modeling these disruptive factors, thus improving the reliability of lung MRI images. The proposed ANN system supports disruptive input variables in correspondence with the output variables that express image quality and reliability. Input variables are taken from clinical data. This tool is intended to be considered as a diagnostic aid in thoracic imaging.
Lung MRI, Proton density, Pulmonary embolism, BOLD signal, Artificial intelligence, Artificial neural networks (ANN), Image quality, Diagnostic reliability, Thoracic imaging
Thoracic imaging plays an essential role in the diagnosis and monitoring of pulmonary diseases. Due to the absence of ionizing radiation, MRI is strongly recommended as an alternative in the case of contraindications to iodinated contrast agents or repeated exposures [3]. Although computed tomography (CT) remains the recommended method for vascular assessment and detection of pulmonary embolisms. This is because it is characterized by its high spatial resolution and speed [4]. The disadvantage of using MRI remains the low proton density of the lungs and physiological movements limit the quality of the images. The accuracy of the BOLD signal is often affected by cardiac and respiratory movements, which affects the accuracy of the diagnosis [5].
To overcome these drawbacks, ultra-short echo time (UTE) sequences have been developed, which allow the acquisition of morphological images with a very short echo time (TE ~ 0.5 ms) and a limitation of the decay of the T2 signal by the non-Cartesian filling of the k-space, often radial, which improves the spatial resolution [6].
Nowadays, different artificial intelligence tools are becoming essential in this field. The use of these tools in thoracic imaging increasingly improves image quality by correcting artifacts and becomes a diagnostic aid tool [7,8]. Despite these advances, pulmonary MRI signals remain characterized by uncertainties and inaccuracies. Hence, this study proposes an attempt to correct and adjust the factors involved in the process by an artificial neural network system to manage these drawbacks.
Modeling the factors affecting the quality of MRI lung images was performed using artificial neural networks.
Based on real-world data, artificial neural networks have the ability to interpret experimental data and solve complex systems such as biophysical processes. This is because these networks mimic natural neural networks.
The network comprises two mapped spaces (input-output). These systems then link inputs and outputs via a transfer function. The essential phase during network operation is learning. Thus, upon reading the input and output variables, the transfer function is modified to remain optimal. The function is adjusted by adapting mathematical coefficients called "weights." This learning phase then consists of optimizing the function so that it remains valid in all scenarios [9]. The proposed network is a multi-layer network (one input layer, one hidden layer, and one output layer) (Figure 1).
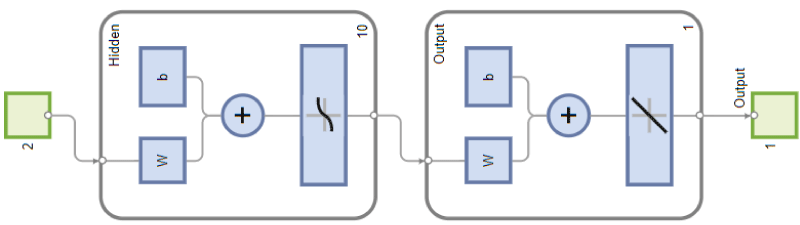 Figure 1: Network architecture.
View Figure 1
Figure 1: Network architecture.
View Figure 1
The network's input and output variables include:
• The degree of inaccuracy of the BOLD signal, influenced by the low proton density and the rapid signal decay, normalized between 0 (maximum accuracy) and 1 (maximum inaccuracy).
• A variable that quantifies the intensity of magnetic disturbances due to cardiac and respiratory movements, on a normalized scale of 0 to 1.
• The output variable corresponds to the degree of reliability of the obtained image, expressing the diagnostic confidence from 0 (unreliable image) to 1 (highly reliable image) (Table 1).
Table 1: Coding of input-output variables. View Table 1
Transfer function optimization is achieved by training the neural network using a set of real clinical cases, integrating radiological expertise and quantitative measurements of MRI signals.
The neural system was integrated downstream of preprocessing to provide a quantitative assessment of image quality. For better diagnostic interpretation and to enable the signaling of acquisitions to be repeated or completed, this assessment serves as a decision-making aid for the radiologist, indicating the optimal images.
Using the neural network to process clinical data across a set demonstrates an improvement in the selection of reliable images. Visual quality remains to be assessed by the expert. Images deemed reliable exhibited better spatial resolution and fewer artifacts, facilitating the detection of pulmonary abnormalities, particularly proximal embolisms [10].
UTE sequences combined with neural network analysis reduce false negatives related to motion artifacts and weak signals. The diagnostic sensitivity of lung MRI is thus improved.
The network's performance is presented in figure 2.
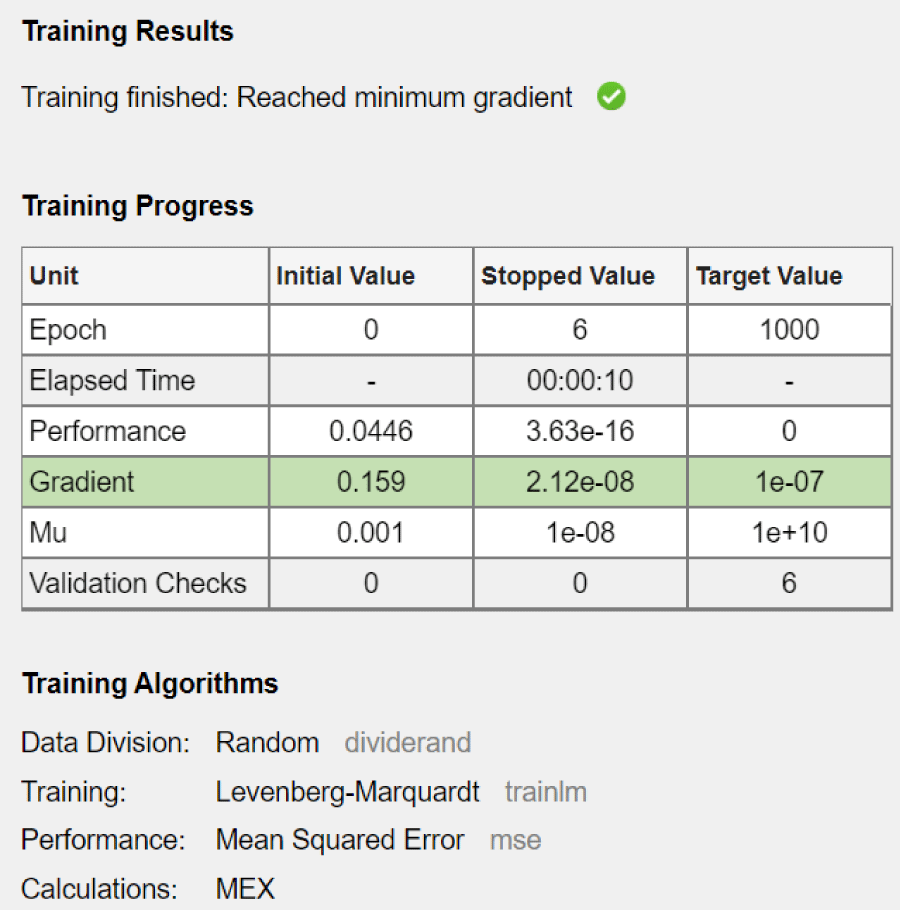 Figure 2: System performance.
View Figure 2
Figure 2: System performance.
View Figure 2
The training process stopped when the minimum gradient was reached. This means that the neural network was successfully trained. The stopping of the variation of the performance function explains acceptable convergence. The network converged after six epocs. This means that the model reached the optimal solution quickly. The network performance, measured by the mean squared error (MSE), decreased dramatically from 0.0446 to 3.63e-16. This significant reduction indicates an excellent fit of the model to the training data. The gradient reached its target value (1e-07), thus confirming the convergence of the model. Validation checks remained at 0, which means that no early stopping conditions based on validation performance were triggered. This is consistent with the observed fast convergence and good performance.
The histogram of the distribution of errors made by the regression model. Ideally, the errors should be centered on zero, which would indicate that the model is making generally correct predictions (Figure 3), but the distribution of errors is not perfectly centered on zero. There is a notable scatter of errors to the left (negative errors) and right (positive errors) of zero. The histogram also shows that the frequency (number of instances) of each error range with the highest bars is close to zero, meaning that the model is making more predictions with relatively small errors than with large errors.
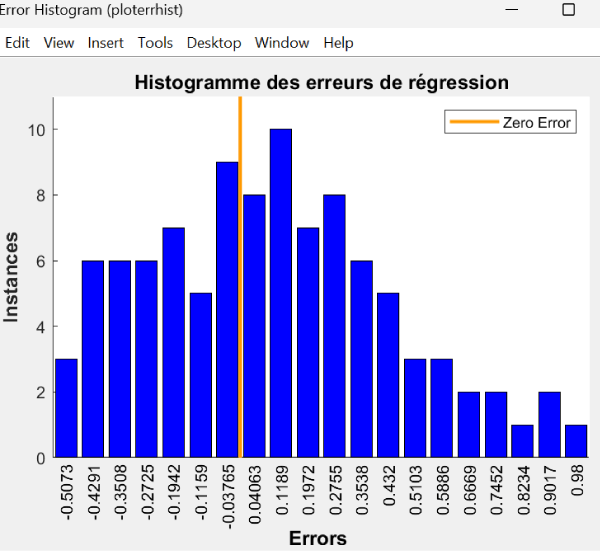 Figure 3: System performance.
View Figure 3
Figure 3: System performance.
View Figure 3
The line in Figure 4, representing the model's learned relationship between target and output values with an equation, (Output ~ = 0.86.Target + 0.17), indicates that for every unit increase in the target, the model's output increases by approximately 0.86 units, with a constant offset of 0.17. This line is close to the dotted line but does not overlap it exactly, meaning the model is capturing the overall trend but with some error. The equation shows that the model is underestimating the slope. A perfect slope would have a value of 1. The bias of 0.17 indicates that the model tends to predict a value that is 0.17 higher when the target is 0.
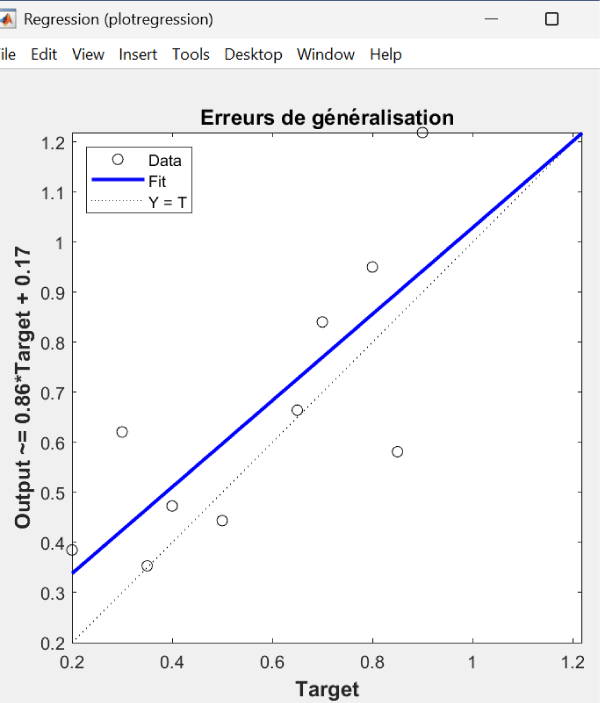 Figure 4: Error generalization.
View Figure 4
Figure 4: Error generalization.
View Figure 4
Improving model performance requires collecting a large amount of data for training, and in this case, it would be wise to try a different network architecture.
In general, lung MRI benefits greatly from technological advances such as UTE sequences and AI algorithms [1,7]. The neural network approach proposed here brings an additional dimension by explicitly addressing the uncertainty inherent in lung MRI signals.
The integration of AI in thoracic imaging is growing rapidly, with applications ranging from noise reduction to automated diagnostic assistance [11,12]. Our neural network system is part of this dynamic, providing decision support based on rigorous uncertainty modeling.
The proposed system adopts artificial neural networks as an artificial intelligence technique for analyzing and processing lung MRI data. The system takes into account uncertainties related to weak BOLD signals and motion artifacts. This model, combined with UTE sequences, opens new perspectives for the clinical use of MRI in the diagnosis of lung diseases, especially when contrast agents are contraindicated. The developed system still suffers from limitations due to the limited sample size analyzed. With the expansion of its database, this tool could be considered a diagnostic aid in thoracic imaging.
All authors declare that there are no conflicts of interest.