Diverse groups of ototoxic agents and gene mutation induce hearing disorders. Several studies showed that increased oxidative stress, inflammation, and glutamate are involved in the initiation and progression of hearing disorders. Therefore, reducing these biochemical defects before and after exposure to ototoxic agents may prevent and improve the management of hearing loss. Previous studies with a single agent have yielded variable benefits in hearing disorders. This review briefly presents evidence for the involvement of oxidative stress, inflammation, and glutamate in the initiation and progression of hearing disorders. It proposes a novel concept that simultaneous elevation of the levels of antioxidant enzymes, dietary and endogenous antioxidant compounds, and reduction in the levels of glutamate may be necessary for the optimal benefits in prevention and improved management of hearing disorders. Supplementation with micronutrients can elevate the levels of antioxidant compounds and reduce the levels of glutamate. However, increasing the levels of antioxidant enzymes is complex requiring an activation of the nuclear transcriptional factor-2 (Nrf2). This review briefly describes the regulation of activation of Nrf2, and proposes a micronutrient mixture that can simultaneously activate Nrf2, enhance the dietary and endogenous antioxidant compounds levels, and inhibit the release and toxicity of glutamate for prevention and improved management of hearing loss.
Oxidative stress, Inflammation, Nuclear transcriptional factor-2 (Nrf2), Hearing disorders, Glutamate
Hearing disorders are caused by diverse groups of ototoxic agents, such as chronic and intense noise, vibrations, gentamicin, ionizing radiation, cisplatin, large doses of aspirin, bacterial and viral infection, gene mutations, and advanced age. In addition, health conditions, such as ear canal obstruction, mechanical damage to the tympanic membrane, and ossicles of the middle ear and inner ear also contribute to the hearing loss. Approximately 48 million Americans have varying degrees of hearing loss. Among individuals of 65 years of age, one of every three people suffers from this disease. Among children, congenital hearing loss is a major hearing disorder that is estimated to be 2-3 cases per 1000 (Hearing Loss Association of America, 2015). Congenital hearing defects could be primarily due to cytomegalovirus infection during pregnancy or gene mutation. Troops in combat zones, musicians or industrial workers are likely to develop varying degrees of hearing loss despite the use of earplugs. Thus, hearing problems represent a major health concern both for the civilian and military in the USA.
Except for ear protective devices, there are no preventive strategies for reducing the risk of noise-induced hearing loss (NIHL). However, despite the use of earplugs, the energy generated from the noise can penetrate the inner ear damaging the hair cells. In order to develop an effective preventive strategy, the major cellular defects that initiate and promote hearing loss should be identified. Several studies of the past decades indicate that increased oxidative stress [1-6], inflammation [7-13], and glutamate [14-16] play a central role in the initiation and progression of hearing defects. Therefore, reducing these biochemical defects simultaneously may be one of the rational strategies for the prevention of hearing loss. Several animal studies [17-24] showed that the use of primarily one antioxidant reduced the risk of developing hearing loss by decreasing oxidative stress; however, in a few human studies, the use of one or a mixture of a few antioxidants or steroid produced variable degrees of reduction in hearing loss with or without standard therapy [25-31]. The exact reasons for this discrepancy between animal and human studies are unknown. It is possible that a single antioxidant alone may not be sufficient to simultaneously reduce oxidative stress, inflammation and glutamate in humans exposed to ototoxic agents. In addition, a single antioxidant in a high oxidative environment found in patients with hearing loss is oxidized and then acts as a pro-oxidant rather than as an antioxidant.
The treatment of hearing disorders that includes steroids [32,33], glutamate antagonist [34,35], various types of hearing aids [36-38], and cochlear implants [39,40]. Although these treatments produce varying degrees of improvements but they are considered unsatisfactory. One of the reasons could be that none of these treatment modalities simultaneously reduce oxidative stress, chronic inflammation and glutamate levels.
A previous study has proposed that simultaneous elevation of the levels of antioxidant enzymes, and dietary and endogenous antioxidant compounds may be necessary for optimally reducing oxidative stress, inflammation and glutamate levels in humans [41]. Supplementation with micronutrients can increase the levels of antioxidant compounds and reduce the levels of glutamate; however, elevating the levels of endogenous antioxidant enzymes is complex that generally requires an activation of the nuclear transcriptional factor-2 (Nrf2).
This review briefly presents evidence for the involvement of oxidative stress, inflammation, and glutamate in the initiation and progression of hearing disorders induced by diverse groups of ototoxic agents. This review briefly discusses the regulation of activation of Nrf2, and proposes a mixture of micronutrients that can simultaneously activate Nrf2, enhance the levels of antioxidant compounds, and reduce the levels of glutamate for prevention and improved management of hearing disorders.
Low levels of reactive oxygen species (ROS) are essential for driving several biochemical reactions necessary for maintaining the cellular function; however, high levels of ROS override cellular defense mechanisms against oxidative damage, and induce cellular injury including damage to DNA, RNA, lipid, and protein. If cellular damage is not fully repaired, chronic inflammation sets in motion, which releases free radicals, pro-inflammatory cytokines, adhesion molecules and prostaglandins, all of which toxic are to the cells. Oxidative damage to the auditory cells causes release of glutamate. Glutamate at high concentration is toxic to the hair cells. Several studies show that increased oxidative stress, chronic inflammation and glutamate play an important role in the initiation and progression of hearing disorders. These studies are described under separate sections.
Exposure to high intensity noise decreased the levels of serum total antioxidant capacity and increased the levels of nitric oxide in guinea pigs [42]. Increased nitric oxide levels can form peroxynitrite that damages the hair cells. Impulse noise exposure also enhanced oxidative stress in some animal studies. The levels of nitric oxide, peroxynitrite, oxidative stress, nuclear factor-kappa-beta (NF-kB), glutamate receptor (N-methyl-D-aspartate), and calcium are elevated in patients with tinnitus [1-3,43-46]. Noise exposure induced approximately 21 to 42% of tinnitus in humans [4,47,48]. About 34% of tinnitus patients had post-traumatic stress disorders (PTSDs) [49]. These studies suggested that some common biochemical defects that cause both tinnitus and PTSD might exist in these diseases. Indeed, a recent review has shown that increased oxidative stress occurs in patients with PTSD and tinnitus [50].
NADPH (nicotinamide adenine dinucleotide) oxidases (NOXs) transport electron across the plasma membrane and produce superoxide radicals from oxygen. Exposure to moderate or intense noise increased NOXs activities in the cochlea of rats. Treatment of rats with diphenyleneiodonium, an inhibitor of NOX, after noise exposure, prevented hearing loss [5]. Pravastatin treatment also decreased NIHL by inhibiting NOX activity in mice [51]. Frequent exposure to vibration also produces hearing disorders. The older guinea pigs were two-fold more sensitive to vibration than younger animals [49]. The combination of noise and vibration during the use of hand-held vibrating tools increased the risk of hearing loss in industrial workers [6,52]. Increased oxidative stress and chronic inflammation are also associated with ageing [53], and age-related cochlear structural alterations and degeneration of sensory and neural cells also occurred [54] that resembles normal aging. Hearing loss occurred due to increased oxidative stress in a model of aging rats. In addition, accumulation of mutated mitochondrial DNA was found in the peripheral and central auditory cells [55-57].
Cisplatin caused hearing loss by generating free radicals. Treatment with this drug significantly depressed the levels of antioxidant enzymes, superoxide dismutase (SOD), glutathione peroxidase, glutathione reductase, glutathione transferase and catalase, and enhanced the levels of lipid peroxidation [58]. Carboplatin treatment likewise decreased glutathione content [59]. Cisplatin treatment also reduced the intracellular levels of nicotinamide adenine dinucleotide (NAD+); thereby, interfered with energy metabolism. Activation of NAD (P) H: quinone oxidoreductase 1 (NQO1) by beta-lapachone that exhibits antioxidant and anti-inflammation activities, protected against cisplatin-induced ototoxicity by increasing the levels NAD+ and sirtuin-1 protein [60].
. A role for oxidative stress in Meniere's disease (MD) is supported by the fact that free radical scavengers such as rebamipide, vitamin C, and glutathione, when administered orally for 8 weeks to patients with poorly controlled MD, improved tinnitus and prevented hearing loss [61].
Inflammation also plays an important role in hearing disorders induced by noise, drugs, and advancing age. Noise exposure can damage cochlear function by inducing inflammation in animal models [7,62]. This is supported by the fact that exposure to noise increased the levels of intracellular adhesion molecules and migration of leukocytes. Intense noise exposure can also activate the nuclear transcription factor-kappaB (NF-kB) in the cochlea of mice that causes over-expression of pro-inflammatory products including intracellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) and inducible nitric oxide synthase (iNOS) in the inner ear that contribute to the hearing loss [8].
Aspirin and other anti-inflammatory drugs prevented gentamicin-induced hearing loss and improved hearing ability [25,63]. Pro-inflammatory cytokines and activation of NF-kB contribute to the cisplatin-induced ototoxicity in mice and immortalized cochlear cells in cuture [9,64]. Tumor necrosis factor-alpha (TNF-α) also induced cochlear degeneration after bacterial meningitis. Administration of TNF-α antibody reduced meningitis-induced hearing loss in Mongolian gerbils [10]. Increased levels of TNF-α were also associated with trauma-induced hearing loss. Treatment with dexamethasone reduced TNF-α-induced damage to the organ of Corti explants cultures [65]. A study has reported that otosclerosis-induced sensorineural hearing loss is due to the chronic release of TNF-α from the foci of otic capsule [11,66]. Chronic inflammatory reactions produce sac dysfunction leading to MD [67]. Increased inflammation in the elder individuals was associated with age-related hearing loss [12] and sensorineural hearing loss [13]. Polymorphism of interleukin-6 (IL-6) increased the sensitivity of noise-induced hearing disorders in individuals over the age of 60 years [68].
When the hair cells are injured, glutamate transmission responsible for converting vibration sound into electrical signal, is enhanced. Glutamate at high concentrations is neurotoxic. Damage to the peripheral auditory and somatosensory systems causes imbalance between excitatory and inhibitory neurotransmitters in the mid brain auditory cortex and brain stem. This imbalance in neurotransmission can cause hyperactivity in the auditory cortex leading to the perception of phantom sounds (tinnitus). Intense noise causes release of excessive amounts of glutamate that damage the inner hair cells-auditory synapses. Kynurenate, a glutamate antagonist, protected guinea pigs against NIHL [14]. The glutamate-aspartate transporter (GLAST) that plays an important role in maintaining the normal levels of glutamate was decreased in the cochlea after exposure to noise leading to increased accumulation of glutamate in the perilymphs. The resulting increased levels of glutamate were associated with an enhanced rate of progression of hearing loss [15]. Treatment with riluzole, an inhibitor of glutamatergic neurotransmission, protected guinea pigs against NIHL [69]. Treatment with D (-)-2-amino-5-phosphonopentanoic acid (D-AP5), a selective inhibitor of glutamate receptor N-methyl D-aspartate (NMDA) receptor, attenuated noise-induced tinnitus [16]. Diagrammatic representation of noise-induced biochemical events leading to hearing loss is represented in Figure 1.
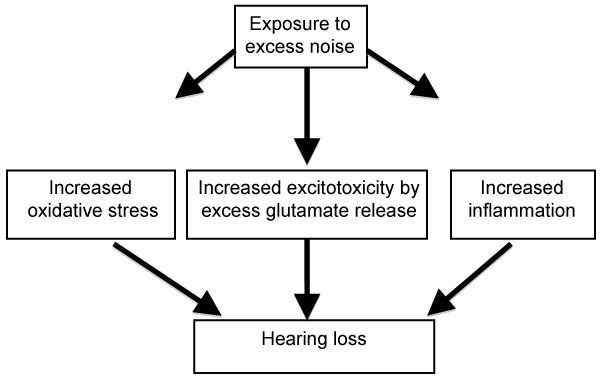 Figure 1: Diagrammatic representation of noise-induced hearing loss pathways. View Figure 1
Figure 1: Diagrammatic representation of noise-induced hearing loss pathways. View Figure 1
Additional pre-clinical and clinical studies should be performed to substantiate the role of oxidative stress, chronic inflammation, and glutamate in hearing disorders, using exposure to noise as an example of the ototoxic agent. The levels of markers of oxidative damage, pro-inflammatory cytokines, and glutamate in the plasma of same subject before and after exposure to noise for the prevention studies, and in combination with standard care, for improving the management of hearing loss studies, should be measured.
The body protects against oxidative damage by antioxidant compounds derived from the diet as well as made in the body, and antioxidant enzymes made in the body. Antioxidant compounds reduce oxidative stress by a mechanism in part different from that of antioxidant enzymes. They destroy free radicals by donating electron to unpaired state of the free radicals. On the other hand, antioxidant enzymes destroy free radicals by catalysis converting them to the harmless molecules (such as to water and oxygen). Some antioxidant compounds also reduce chronic inflammation [70-74]. Different antioxidants have different affinities for free radicals depending upon their location in the cellular compartments. Water-soluble antioxidants such as vitamin C and glutathione protect molecules in the aqueous environment of the cells, whereas lipid soluble antioxidants such as vitamin A and vitamin E protect molecules in the lipid environment of the cells. Vitamin E was more effective in quenching free radicals in a reduced oxygenated cellular environment, whereas vitamin C and vitamin A were more effective in a higher oxygenated environment of the cells [75]. Vitamin C is important for recycling the oxidized form of vitamin E to the antioxidant form, [76]. In addition, antioxidants produce cell protective proteins by upregulating or down regulating different microRNAs [77]. For example, some antioxidants can activate Nrf2 by upregulating miR-200a that inhibits its target protein Keap1 [78], whereas others activate Nrf2 by downregulating miR-21 that binds with 3-UTR Nrf2 mRNA [78].
Vitamin E treatment reduced cochlear damage in NIHL [17,79], and in hearing loss induced by cisplatin [18,80] and gentamicin [81,82]. Alpha-lipoic acid treatment protected against NIHL and carboplatin-induced hearing loss in animals [19,83,84]. The role of N-acetylcysteine (NAC) in reducing NIHL has been reviewed [42]. Treatment with (NAC) significantly reduced noise-induced hair cell loss in cochlear cells of rats and guinea pigs [20]. A combination of acetyl-L-carnitine and NAC also provided protection against hearing loss induced by noise, cisplatin, and aminoglycoside [85,86].
Coenzyme Q10 [21], Idebenone, a synthetic analog of coenzyme Q10 [87], and the soluble form of coenzyme Q10 were effective in reducing hypoxia-induced hearing loss and in NIHL [88]. The combination of vitamin E and idebenone was additive in protecting against NIHL, suggesting that these two antioxidants act by different mechanisms [89]. Oral administration of MitQ, a mitochondria-targeted derivative of ubiquinone, reduced gentamicin-induced hearing disorders by protecting the cochlea [22].
Vitamin C also protected against NIHL in albino guinea pigs [23]. D-methionine attenuated noise-induced oxidative stress and functional loss in the cochlea of mice [90]. Treatment with resveratrol reduced NIHL by decreasing cyclooxygenase-2 (COX-2) levels [24]. Folic acid deficiency induced premature hearing loss by increasing the levels of oxidative stress and homocysteine in mice [91]. Indeed, supplementation with folic acid reduced hearing disorders by reducing oxidative stress and homocysteine levels [92].
Caloric restriction and treatment with individual antioxidants, such as vitamin E, vitamin C, acetyl-L-carnitine, alpha-lipoic acid and melatonin improved auditory sensitivity to sound, reduced mitochondrial DNA deletion and loss of hair cells in aging rats [93-95]. In a mice model of premature aging, age-related hearing loss occurred. Treatment with n-acetyl-L-carnitine failed to protect against hearing loss in these animals [96]. Taken together these reports show that a marked degree of protection against NIHL can be attained by a variety of antioxidants and related compounds.
In a prospective randomized study, intravenous administration of magnesium sulfate improved hearing recovery in patients with idiopathic sudden sensorineural hearing loss [97]. Coenzyme Q10 delayed the progression of hearing loss in patients with a genetic defect, 7445A→Gmitochondrial mutation [26]. The use of glutamate antagonists, steroids and antioxidants may be useful in the management of hearing loss and tinnitus [98]. An oral supplementation with antioxidants (vitamin E, vitamin C, beta-carotene and phospholipids) reduced the subjective discomfort and tinnitus intensity in patients with idiopathic tinnitus [27]. NAC protected against aminoglycoside-induced ototoxicity in hemodialysis patients [99]. An antioxidant mixture containing reduced glutathione, alpha-lipoic acid, cysteine, and other antioxidants improved the symptoms of MD [100]. A combination of vitamins A, C and E, and selenium in combination with standard therapy improved hearing gain more that that produced by standard therapy alone in patients with idiopathic sensorineural hearing loss [28]. Intravenous administration of high dose vitamin C in combination with steroid therapy improved hearing gain more than that produced by steroid therapy alone [29]. Vitamin E supplementation and carbogen (5% CO2 + 95% O2) breathing reduced noise-induced hearing loss [30]. A commercial preparation of multiple micronutrients reduced symptoms of tinnitus in humans [31].
In order to optimally reduce oxidative stress and inflammation in post-traumatic disorders (PTSDs) and traumatic brain injury (TBI), simultaneous elevation of the levels of antioxidant enzymes through an activation of the Nrf2/ARE (nuclear transcriptional factor-2/antioxidant response element) pathway, as well as enhancing the levels of dietary and endogenous antioxidant compounds may be essential [50]. A similar strategy may be needed for reducing hearing loss in humans.
Since an elevation of the levels of antioxidant enzymes and other cytoprotective enzymes requires activation of Nrf2, this factor is briefly reviewed. The nuclear transcriptional factor, Nrf2 (nuclear factor-erythroid-2- related factor 2) belongs to the Cap 'N'Collar (CNC) family that contains a conserved basic leucine zipper (bZIP) transcriptional factor [100]. Under physiological condition, Nrf2 is associated with Kelch-like ECH associated protein 1 (Keap1), which acts as an inhibitor of Nrf2 [101]. Keap1 protein serves as an adaptor to link Nrf2 to the ubiquitin ligase CuI-Rbx1 complex for degradation by proteasomes and maintains the steady levels of Nrf2 in the cytoplasm. Nrf2-keap1 complex is primarily located in the cytoplasm. Keap1 acts as a sensor for ROS/electrophilic stress.
Normally, during acute oxidative stress, ROS is needed to activate Nrf2 which then dissociates itself from Keap1- CuI-Rbx1 complex and translocates in the nucleus where it heterodimerizes with a small Maf protein, and binds with the ARE (antioxidant response element) leading to increased expression of target genes coding for several cytoprotective enzymes including antioxidant enzymes [102-105]. Thus, activation of Nrf2 during acute oxidative stress is likely to protect the cochlea from oxidative damage.
Activation of Nrf2 alone may not be sufficient to increase the levels of antioxidant enzymes. Activated Nrf2 must bind with antioxidant response element (ARE) in the nucleus for increasing the expression of its target genes. This binding ability of Nrf2 with ARE was impaired in aged rats and this defect was restored by supplementation with alpha-lipoic acid [106]. It is unknown whether the binding ability of Nrf2 with ARE is impaired in hearing disorders.
Activation of Nrf2 by ROS becomes impaired during chronic oxidative stress found in patients with hearing loss [107-109]. This is evidenced by the fact that increased oxidative stress occurs in hearing disorders despite the presence of Nrf2.
Although changes in the expressions of microRNAs are being investigated in hearing disorders, this section focuses on the role of microRNAs in regulating Nrf2 activation. microRNAs (miRs) are evolutionarily conserved small non-coding single-stranded RNAs of approximately 22 nucleotides in length, and are present in all living organisms including humans [110-113]. Each micro RNA binds to its complimentary sequences in the 3'- untranslated region (3'-UTR) of the target mRNA that prevents the formation of its protein, and thus, plays an important role in regulating cell function. It appears that specific microRNAs may regulate the activation of Nrf2 by decreasing the levels of Keap1. The complex of Keap1-Nrf2 in the cytoplasm prevents activation of Nrf2. Over-expression of miR-200a reduced Keap-1 levels allowing Nrf2 to migrate to the nucleus where it binds to the ARE that enhanced the transcription of target cytoprotective genes including antioxidant enzymes [114]. Increased oxidative stress associated with hearing disorders enhanced the expression of miR-153 and reduced the expression of Nrf2 by binding to its 3'-UTR region of Nrf2 mRNA [115]. Mutation in miR-153 restored the oxidative stress-induced reduction in Nrf2 activity.
During chronic oxidative stress, activation of Nrf2 becomes unresponsive to ROS. Antioxidant compounds activate Nrf2 by a ROS-independent mechanism. Some of them are listed here.
Some examples are vitamin E and genistein [116], alpha-lipoic acid [106], curcumin [117], resveratrol [118,119], omega-3-fatty acids, glutathione [120,121], glutathione [122], NAC [123], coenzyme Q10 [124], and several plant-derived phytochemicals with antioxidant activities, such as epigallocatechin-3-gallate, carestol, kahweol, cinnamonyl-based compounds, zerumbone, lycopene and carnosol [125,126], genistein [116], allicin, a major organosulfur compound found in garlic [127], sulforaphane, a organosulfur compound, found in cruciferous vegetables [128], and kavalactones (methysticin, kavain and yangonin) [129]. The steps whereby ROS-independent activation of Nrf2 may be protective against hearing loss are depicted in Figure 2.
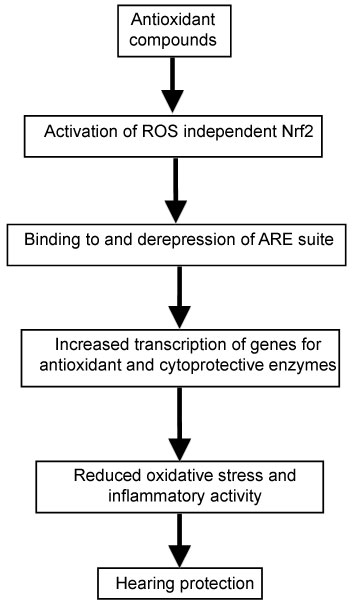 Figure 2: Proposed pathway of protection against hearing loss by antioxidant micronutrients. View Figure 2
Figure 2: Proposed pathway of protection against hearing loss by antioxidant micronutrients. View Figure 2
L-carnitine activates Nrf2 by a ROS-dependent mechanism [130], probably by generating transient ROS.
Activation of Nrf2 may not be sufficient to optimally reduce oxidative stress, because antioxidants compounds are also decreased during chronic oxidative stress [131-133]; therefore, their levels must also be simultaneously elevated.
Activation of Nrf2 [134,135] and some individual antioxidant compounds reduced chronic inflammation [70-74,136,137].
In Nrf2 deleted mice (-/-) hair cells become more sensitive to noise-induced oxidative damage in comparison to wild-type mice. Pre-treatment with an activator of Nrf2 preserved integrity of hair cells and improved hearing levels in wild-type mice but not in Nrf2 deleted mice (-/-). These results suggest that high activity of Nrf2 in chochlear protects against noise-induced hearing loss [138].
Cisplatin can cause hearing loss by increasing levels of oxidative damage and pro-inflammatory cytokines in hair cells. Pre-treatment with an activator of Nrf2, such as Erdosteine [139], flunarizine [140], Ebselen [141], Ginkgolide B, a major component of Ginkgo biloba [142], and phloretin [143] protected hair cells (HE1-OC1) by reducing oxidative damage, pro-inflammatory cytokines, inhibition of pro-apoptotic expression, and enhancing the levels of cytoprotective enzymes including antioxidant enzymes.
Increased oxidative stress contributes to cochlear damage, including age-related hearing loss and gentamicin-induced ototoxicity. The Nrf2-knockout mice maintained normal auditory thresholds at an their age of 3 months; however, their hearing ability was significantly impaired as they grow older (6 and 11 months) in comparison to age-matched wild type mice [144]. In addition, the number of hair cells and spiral ganglion cells was markedly decreased in Nrf2-knockout mice. In the explants culture of Corte, treatment with gentamicin ehanced the loss of hair cells in Nrf2-deficient tissue more than that found in wild-type mice. Activation of Nrf2 occurred only in the wild-type mice, but not in Nrf2-knockout mice. These results further suggest importance of Nrf2 in the auditory for protection of hearing disorders.
Some antioxidants decrease the release of glutamate as well as its neurotoxicity [145-147]. In addition, certain B-vitamins can also decreased the release of glutamate [148,149].
Because of failure to produce consistent benefit in improving the symptoms of hearing disorders with one or two micronutrients, a comprehensive micronutrient mixture is proposed. This mixture contains multiple dietary antioxidant compounds (vitamin A, natural mixed carotenoids, vitamin C, vitamin D, vitamin E, curcumin, resveratrol), endogenous antioxidants (alpha-lipoic acid, L-carnitine, and coenzyme Q10), and a synthetic antioxidant N-acetylcysteine (NAC), omega-3-fatty acids, and all B-vitamins. This mixture of micronutrients may optimally reduce oxidative stress and chronic inflammation by simultaneously enhancing the levels of antioxidant enzymes through activation of the Nrf2/ARE pathway, and elevating the levels of antioxidant compounds. The same micronutrient mixture may also reduce the release and toxicity of glutamate.
The purpose of primary prevention is to protect healthy individuals from developing hearing disorders. Older individuals, musicians, industrial workers, troops who are likely to be employed in the war zones, individuals who are likely to receive certain antibiotics for their health conditions, and individuals carrying mutations in genes responsible for hearing loss, are suitable subjects for the primary prevention studies.
At present, there are no strategies to delay the onset of hearing loss in individuals with mutated genes. The proposed mixture of micronutrients may be effective in preventing or delaying the onset of symptoms of hearing disorders in these individuals. This possibility is indirectly supported by an experiment on the fruit flies described here.
The gene HOP (TUM-1) is essential for the development of Drosophila melanogaster (fruit fly). A mutation in this gene markedly increases the risk of developing a leukemia-like tumor in female flies. In collaboration with Dr. Bhattacharya of NASA Moffat Field, CA, we observed that whole-body irradiation of these flies with proton radiation dramatically increased the incidence of cancer compared to that observed in un-irradiated female flies. Treatment with a mixture of multiple antioxidants before and after irradiation blocked the incidence of proton radiation-induced cancer in female fruit flies [150].
The purpose of secondary prevention is to stop or slow the progression of hearing disorders after exposure to ototoxic agents. Individuals who have been exposed to such agents, but have not developed any hearing problems, and are not taking any medication, are suitable subjects for the secondary prevention of hearing disorders. The micronutrient mixture suggested for the primary prevention studies is also proposed for the secondary prevention studies.
The patients with hearing disorders who are receiving standard care are suitable for this study. The micronutrient mixture suggested for the primary prevention studies is also proposed in combination with standard care for the improved management studies.
Published studies suggest that increased oxidative stress, chronic inflammation, and glutamate play a key role in the initiation and progression of hearing disorders, irrespective of the types of ototoxic agents or health conditions. Some antioxidant compounds used in animal and human studies to prevent or treat hearing loss may activate Nrf2, but this may not be sufficient to optimally reduce oxidative stress, chronic inflammation, and glutamate release. This may be due to the fact that the levels of dietary and endogenous antioxidant compounds are also depleted in a high oxidative environment found in patients with hearing disorders. Their levels cannot be increased by supplementation with one or two antioxidant compounds. The proposed micronutrient mixture may serve to optimally reduce oxidative stress and chronic inflammation by simultaneously enhancing the levels of antioxidant enzymes through activating the Nrf2/ARE pathway, and dietary and endogenous antioxidant compounds. Such a micronutrient mixture may also reduce the release and toxicity of glutamate. The efficacy of this mixture of micronutrients should be tested in the primary prevention, secondary prevention, as well as in combination with standard care in improved management studies.
The author is a Chief Scientific Officer of Engage Global, Provo, Utah.
The author thanks Professor Stephen C. Bondy, University of California, Irvine, for reviewing and editing this manuscript.
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sector.