Diagnosis and treatment of Charcot-Marie-Tooth (CMT) can present as a challenging issue. Several published studies have demonstrated the complexity of diagnosis and treatment with an increasing role of genetics in diagnosis. The goal of this review is to contribute an updated and concise overview to the limited body of literature. This review elaborates on the published data with regard to discussing the presence of CMT disease and manifestation of various foot and lower extremity deformities including reported treatment along with presentation of a single case report. In our review, CMT demonstrated a greater incidence of foot deformities such as pes cavus and claw toes. A reduction in normal walking ability due to progressive muscle weakness, starting from the intrinsic muscles and moving to the extrinsic muscles of the leg, was reported in the lower extremity. CMT has shown to severely impact quality of life both physically and psychologically. Genetic screening is playing an ever-increasing role in the diagnosis of CMT; however, electromyography (EMG) and nerve conduction velocity (NCV) still play a heavy role in diagnosis. Surgical treatment is discussed in depth along with a treatment algorithm. More research is warranted in regard to genetic screening, diagnosis, prevention, and treatment of the various sequela to improve the quality of life for those with CMT.
CMT, Gait analysis, Hereditary Motor and Sensory Neuropathy (HMSN), Foot drop, Pes cavus, Cavovarus, Claw toes
Charcot-Marie-Tooth Disease (CMT) is a genetic disorder which affects sensory and motor nerves and subsequently has a huge impact on the quality of life of those affected by this disease. The disease was first identified in 1886 by Jean-Martin Charcot and Pierre Marie in Paris, France, and simultaneously but separately by Howard Henry Tooth in Cambridge, England [1]. CMT is considered to be the most commonly inherited neuromuscular disorder with a prevalence of the disease being reported in 1 of 2500 individuals [2,3]. CMT may first appear in infancy with signs of clumsiness or abnormal gait due to foot deformity and muscular atrophy [4]. The main clinical features typical of CMT foot deformities are a high arch (pes cavus), hammertoes, and claw toes. Pes cavus is defined by excessive elevation of the longitudinal arch, secondary to plantar flexion of the first ray, forefoot pronation and adduction, and compensatory hindfoot varus; all of which limit the amount of weight that the middle of the foot bears.
Hereditary peripheral neuropathies are traditionally subdivided into 4 groups: Hereditary sensory and autonomic neuropathy (HSAN), hereditary motor neuropathy, hereditary sensory neuropathy, and a combination of hereditary motor and sensory neuropathy (HMSN). Historically speaking, the aforementioned hereditary peripheral neuropathies were designated by eponyms with specific clinical features, e.g., Charcot-Marie-Tooth being one of the more common HMSNs. While technically more accurate to designate as HMSN, the eponym, Charcot-Marie-Tooth (CMT), has made a resurgence and has generally been accepted as being synonymous with HMSN [4].
CMT represents a spectrum of disorders caused by mutations predominantly expressed in the proteins of myelin and/or the axons of peripheral nerves. A variety of mutation types for CMT have been described including point mutations and whole gene duplications or deletions. Extensive genetic heterogeneity is seen with inherited peripheral neuropathies, meaning patients with identical phenotypes may harbor mutations in different genes, and also a mutation in one gene can lead to a variety of phenotypes [4,5]. Adding to the confusion of CMT are the multiple classifications and naming systems. The traditional naming system was based on nerve conduction velocities and had 2 types: CMT1 or demyelinating (NCV < 38 m/s) and CMT2 or axonal (NCV > 38 m/s) [6,7]. Multiple other classifications have been described, however an alpha numeric system in which 5 types (1- 4 and X) based on clinical picture, often defined by inheritance pattern and/or nerve conduction velocities, has been commonly used. In this system, CMT1 is an autosomal dominant inherited demyelinating form, CMT2 is a dominantly-inherited axonal form, CMT3 is known as Dejerine-Sottas syndrome (severe form with onset in infancy), CMT4 are varying autosomal recessive forms, and CMTX are inherited in an X-linked manner (Table 1). Within each category, a specific letter is assigned with a particular gene or group of affected genes (e.g., CMT2A, CMT2B, etc.) [8]. The two most common types are CMT1 and CMT2; Barreto, et al. performed a systematic review in which they demonstrated a worldwide frequency of the main types being up to 84% for CMT1 and between 12 - 35.9% for CMT2 [9]. In 2018, Magy, et al. proposed a new gene based classification system, with the added benefit of correlating mode of inheritance and neuropathy type with a gene [1]. Currently there are 80+ identified causative genes behind CMT, each with various phenotypic expressions, however describing each is beyond the scope of this article [10].
Table 1: Commonly accepted classification of CMT based on type with descriptive features. View Table 1
Future CMT research has focused on better treatment, understanding, and prevention of the disease. Further identification of genes and of the proteins responsible for CMT can give researchers better understanding of the disease, and perhaps better forms of treatment in the future. The purpose of this article is to provide an updated overview on diagnosis and surgical treatment of lower extremity deformities associated with CMT. A surgical algorithm is provided for treatment. Additionally, a case example is presented of a patient who initially presented with clinical and diagnostic findings consistent with CMT, who underwent successful reconstructive surgery.
The present study was approved by the Institutional Review Board at Georgetown University (Washington, DC). This review elaborated on the epidemiology, etiology, diagnosis of the various deformities manifesting in the lower extremities, and presentation of a single case report. Electronic databases PubMed, ScienceDirect and Cochrane review were used to obtain the data for searches. Keywords utilized in searches were "Charcot Marie Tooth'', "pes cavus", "abnormal gait", and "genetics". Inclusion criteria for this study were articles pertaining to the evaluation, diagnosis and treatment of pes cavus, abnormal gait, and hammertoes in populations with Charcot Marie Tooth disease. Exclusion criteria included genetic based studies greater than 20-years-old and studies without the presence of CMT with occurrence of pes cavus, hammer toes, foot drop, or other notable mention of deformity. Articles containing this information were obtained from years dating back to 1983 to this current year. After inclusion and exclusion criteria were applied, the information within these studies were reviewed to present the common lower extremity deformities and treatment pertaining to CMT.
A comprehensive chart review was performed on a patient who presented at a clinic facility for bilateral foot pain with previously diagnosed CMT who underwent surgical correction of structural foot deformities at a tertiary care facility. The patient had greater than 1 year follow up and post-operative outcomes including wound healing, bone healing, and functionality were documented.
Our literature search revealed 25 studies which met inclusion and exclusion criteria. These various studies were conducted for a number of reasons including diagnosis and the role of genetics, lower extremity manifestations including: gait analysis and structural deformities, diagnostic studies and/or radiographic imaging, and treatments.
With the increasing role of genetics in healthcare, it comes as no surprise that genetics can have a part to play in diagnosing and treating CMT. Diagnostic evaluation of CMT still heavily relies on electromyography (EMG) and nerve conduction velocity (NCV) studies with genetic testing primarily being used as a confirmatory test. CMT1 typically presents with a demyelinating phenotype and has a nerve conduction velocity < 38 m/s. On the other end of the spectrum, CMT2 is typically an axonal pathology with nerve conduction velocities being > 38 m/s [11]. Type 1 is an autosomal dominant form associated with four genes: PMP22, MPZ, LITAF, and EGR2. The number of genes involved dictates how severe the phenotype will be in the patient [2]. CMT2 is subdivided by specific gene affected, with the most common form, CMT2A, being caused by a mutation in the MFN2 gene [12].
Family history and physical examination can be the start of clinical evaluation followed by EMG and NCV testing. Sural nerve biopsy can be useful in some cases, however rarely performed today; it may provide some utility with atypical presentations or equivocal EMG and NCV results [13]. EMG and NCV studies can help classify the subset of the disease and help in distinguishing genotypes involved [3]. As previously mentioned, CMT1 often demonstrates NCV of < 38 m/s [2], whereas CMT2, which is the axonal form, is associated with normal nerve conduction velocity but demonstrates reduced muscle action potential [3]. In CMT2, there is a more balanced atrophy of the muscles which leads more often to a pes planovalgus deformity rather than a cavovarus deformity [14]. Although there is little research looking into the prevalence of these deformities amongst CMT patients, Wines et al. in 2005 found that of the 104 feet they evaluated, 22 were associated with patients diagnosed with CMT Type 2. Of these feet, 55% had a pes planovalgus deformity, 36% had a pes cavovarus deformity, and 2 feet had no deformity [15].
The authors advocate for referral to a neurogenetics center or genetic counselor specializing in neurogenetics. The following protocol is recommended by GeneReviews:
Step 1: Single gene testing for PMP22 duplication/deletion as first line testing. Greater than 50% of CMT is secondary to PMP22 duplication. Should PMP22 duplication/deletion testing be normal, reflex to step 2 [6].
Step 2: Multigene testing for the seven next most commonly involved genes (i.e., GDAP1, GJB1, HINT1, MFN2, MPZ, SH3CT2, and SORD) is most likely to be revealing of underlying genetic cause at the most reasonable cost. Should that be unrevealing, reflex to step 3.
Step 3: Comprehensive genomic testing may be warranted. Exome sequencing is often used, however genomic sequencing is possible.
Patients with CMT often develop contractures, typically from weakened muscles that lift the foot and ankle leading to muscles that curl the foot downward to become tightened and contracted. This results in a cavovarus deformity, which may be characterized by plantar flexion of the first metatarsal, adducted forefoot, claw toes, cavus, and hindfoot varus. The following section will delineate those findings that may be seen within this cohort.
Although gait abnormalities often present early in the disease process, progression of deformity with characteristic changes to the forefoot, midfoot, and hindfoot develops collectively, often as pes cavus [16]. In order to understand the pathogenesis of pes cavus, Sabir, et al. studied 22 persons from a single kinship with CMT [17]. They noted that all patients presented with a pes cavus deformity. The researchers also noted that the cause was due to the atrophy of the intrinsic foot muscles which progressively lead to further soft tissue and bone deformities.
Several articles had an emphasis on understanding the effects of CMT on gait, meaning the patient's pattern of walking. The main purpose of those studies was to understand the walking ability and the various muscular dysfunctions present in a wide range of patients. A gait analysis study, reported in 2007, was conducted on 21 patients with CMT by Don, et al. The study tested the speed, muscle function, and the various movements at the ankle and subtalar joints. This study further divided the patients on the severity of the disease while comparing them to a control group. They noted 16 patients presented with weakness in dorsiflexion (DF), and out of the 16, 10 patients also presented with weakness of plantar flexion (PF). Out of the 21 patients, 20 patients presented with a severe form of foot drop. At the subtalar joint, 17 out of the 21 patients displayed an increase in eversion [18]. A study conducted by Vinci, et al., in 2002, reported that all 64 (128 feet) patients with CMT showed some sign (mild to severe) of foot drop, but only 21.4% of patients also presented with signs of plantar flexion failure. It was also noted that out of the 128 limbs, 110 limbs showed signs of foot inversion [19]. A more recent study reported in 2007 by Newman, et al. was conducted on 16 patients diagnosed with CMT to evaluate joint function during gait. CMT patients in Newman's study displayed distal weakness with variable weakness proximally. 14 out of the 16 patients displayed foot drop during the swing phase of the gait cycle and 11 out of 16 displayed failure of plantar flexion during toe-off. Newman also observed that 11 patients displayed an increase in foot supination [20]. Along with the study of the joints at the ankle, they observed changes at the hip (excessive external rotation and decreased adduction) and knee joint (excessive internal rotation and hyperextension), but to a lesser extent.
The indication of difficulty in acquiring normal gait often starts from an early age, along with other foot deformities [4]. Often, the most pronounced gait abnormality is foot drop and the most pronounced deformity is an increase in inversion. Slow progression of motor and sensory dysfunction first presents itself in the distal portions of the lower extremity later leading to proximal defects [21]. Gait is complicated by the characteristic appearance of foot drop, the inability of lifting the foot during the swing phase and advancing the foot forward [18]. This is likely due to the significant difference in the angles of dorsiflexion and plantar flexion compared to the control groups [20]. It has also been noted that in patients with CMT, dorsiflexion has decreased during swing phase along with an increase in plantar flexion. During the swing phase, normally a patient is able to stabilize and activate upward motion of the foot as needed, due to normal neuromuscular function. Within CMT, initial deficits are often a loss of the function of the peroneal nerve, leading to muscular defects of the dorsiflexors of the foot. During the period of push-off, patients can often have a reduction in plantar flexion. However, this reduction in plantar flexion often presents mid to late in the disease process and is mainly due to the weakness in the function of the gastroc-soleus complex [19,20]. Failure of dorsiflexion leads to compensatory changes to prevent the foot from dragging on the ground while trying to walk, i.e.; a steppage gait pattern with excessive hip and knee flexion may be seen. Failure of plantar flexion will affect the ability to push-off, as the heel is in contact with the ground into late stance, and there is often difficulty moving forward to make the next step.
In addition to the changes in motion at the ankle joint, the motion at the subtalar joint, namely supination and pronation, are often dramatically changed. The ability to evert or pronate the foot has been reduced, resulting in an increase in supination of the foot at the subtalar joint. The display of inversion is passive due to the failure of eversion. The motions at the hip joint and knee joint are also affected to a lesser extent. An increase in hip flexion and knee flexion are considered to be compensatory techniques for the dysfunction at the ankle [21]. Newman, et al. has reported consistent presence of increase in hip external rotation and knee internal rotation throughout the gait cycle in these cohorts.
Along with flexor and extensor defects, the amount of energy needed for the ability to walk has increased. Patients with CMT have been analyzed based on their ability to walk slowly, normal pace, and at a faster pace. It was noted that there was a significant difference in effort to be able to walk fast and at a normal pace compared to the control group's ability of walking at the same pace. The patients' ability to walk faster and at a normal pace than the control group had decreased, but very little difference was noted in the 2 groups in their ability to walk at a slow cadence [22]. The overall defect in gait was through decrease in swing velocity, wider gait, and a short stepping length [18]. CMT plays a major role in changing the walking ability at a young age and progressively changing at an older age, depending on type and severity.
Pes cavus is one of the major phenotypic expressions of CMT and is associated with the most common form of CMT, namely, CMT1A [23]. Pes cavus in CMT patients often present with weakness to the anterior musculature, namely the tibialis anterior, and peroneals. As a result, supplemental extensor tendons, such as extensor hallucis longus and extensor digitorum longus are substituted as secondary ankle dorsiflexors which lead to the claw toe deformity observed in most patients.
The plantar fascia is attached to the calcaneus and metatarsophalangeal joints. During the push-off phase of gait, the toes are extended. This increased tension shortens the distance between the calcaneus and metatarsals causing the medial longitudinal arch to elevate. Hicks described this as "the windlass effect" [24]. Patients with pes cavus often develop a plantarflexed first ray, which along with a high arch, places a continuous tension on plantar fascia resulting in forefoot adduction and hind foot varus.
In the normal foot; the heel, first metatarsal head and fifth metatarsal head form a balanced "tripod" with all three points on the ground during stance phase. If the forefoot is in rigid pronation along with plantar flexion of the first ray, then the flexible hindfoot has to assume a varus position as a compensatory mechanism. This phenomenon has been well-described by Paulos, et al. as the "tripod effect" [25]. This is also the basis for the Coleman block test, as a means to determine whether the hindfoot is rigid or supple, particularly in a varus foot type.
Multiple theories have been proposed for the pathogenesis of pes cavus. Sabir and Lyttle described intrinsic muscle imbalances causing an elevated arch [17]. On the other hand, it was noted that there was limited evidence for a significant role of the intrinsic muscles and whether intrinsics alone would have the capacity to inflict such change. Other theories include the extrinsic musculature and a combination of the intrinsic and extrinsic muscles being causes of the imbalance [26,27].
Mann described the pathogenesis of pes cavus in patients with CMT disease. He described an agonist and antagonist model for how the muscles determine the deformity. Often in CMT, the anterior tibialis muscle and the peroneus brevis muscle develop weaknesses. Antagonist muscles, such as tibialis posterior and peroneus longus, pull harder than the other muscles, causing a deformity. Specifically, the peroneus longus pulls harder than the weak anterior tibialis muscle, causing plantarflexion of the first ray and forefoot valgus. The tibialis posterior pulls harder than the weak peroneus brevis, causing forefoot adduction. Intrinsic muscles of the foot develop contractures while the long extensor to the toes is recruited to assist in ankle dorsiflexion, causing cock-up or claw toe deformity. With the forefoot valgus and the hindfoot varus, increased stress is placed on the lateral ankle ligaments and instability can occur [26]. An understanding of the muscles involved and the sequence of the involvement helps in understanding the deformity.
Most recent studies on CMT suggested that pes cavus is an early and age-dependent manifestation of a CMT1A duplication. Selective denervation of intrinsic foot musculature, particularly of the lumbricals, seems to be the initial mechanism causing cavus deformity, leading to reduced ankle flexibility via compensatory mechanisms [23].
Claw Toe deformity is defined as a condition where there is dorsiflexion at the metatarsophalangeal joint with plantar flexion at both interphalangeal joints. Additionally, the flexor muscles will tend to contract in an effort to allow weight bearing to the digits. This causes further clawing of the toes which results in continued deficit by placing more pressure on the windlass mechanism, leading to further metatarsal plantar flexion and exacerbation of the pes cavus deformity. These deformities are also accompanied not only by motor loss, but also sensory loss resulting in neuropathy in the hands and feet and increased incidence of falling and tripping [3,4,28].
Claw toe deformity in CMT is an associated deformity involving intrinsic musculature and/or long flexors and extensor muscles. Balance in the toes results from the modifying force of the intrinsic musculature to flex the metatarsophalangeal joints and extend the interphalangeal joints. When function of the intrinsic muscles is lost, as characteristically occurs in CMT1A, the unopposed force of the extrinsic muscles dorsiflexes the metatarsophalangeal joints and flexes the interphalangeal joints, resulting in a clawfoot deformity [23].
Gallardo using MRI demonstrated selective involvement of intrinsic foot muscles as the characteristic pattern of CMT-1A cases with minimal disease signs. Afterwards this pattern usually combines variable involvement of leg muscles. These findings help to clarify the pathogenesis of pes cavus in the disease [29].
In addition to the muscle imbalances described above, a number of factors change the shape and structure of the foot that contribute to pes cavus. Initially, contracture of the plantar fascia was considered to be an important mechanism to change the shape of foot, but the work of Paulos, et al. pointed towards the interaction between forefoot pronation and hind foot varus, which seemed to be the most important factor in adult patients.
When the claw toe deformity becomes severe, the planter plate ruptures and the digit is dorsally displaced. The planter plate is still tethered to deep transverse metatarsal ligaments and as the slips of the ligament tighten they will cause the metatarsal head to depress by a plunger effect.
Plain film radiographs can be a powerful tool in assessing severity of deformity and preoperative planning. Hibbs angle, or the bisection of the long axis of the first metatarsal and calcaneus on a lateral projection, increases with increasing deformity, and an angle greater than 45 degrees is suggestive of pes cavus. Lateral Meary's angle, which consists of a bisection of the talus and 1st metatarsal, increases proportionally to the severity of cavus deformity. Normally the midline of the first metatarsal and talus are in line with each other, in cavus feet with an angle of greater than 4 degrees, with the convexity superior, correlates with pes cavus. Lateral calcaneal inclination increases with cavus deformity, with normal being 18 to 20 degrees. On an anterior-posterior (AP) view the talocalcaneal angle decreases with hindfoot varus seen in CMT. The talonavicular coverage angle increases with the cavus attitude of the foot. A long leg calcaneal axial has the benefit of allowing the leg-rearfoot-forefoot relationship to be assessed [30,31].
Berciano, et al. conducted clinical, electrophysiological and MRI studies in 11 patients with CMT1A in a wide age range of patients. They agreed with studies of other researchers that involvement of intrinsic muscles of the foot, particularly the lumbricals, to be the initial pathology leading to pes cavus deformity but in advanced stages of disease, this pattern becomes mixed with involvement of distal leg muscles [23] which is supported by the aforementioned study by Gallardo [29].
CMT currently has no cure. Based on the authors experience, careful examination of the patient is critical to identifying deformity and determination of whether it is reducible or rigid. Accurate assessment will help guide treatment options. Patients can often lessen the severity of their symptoms through physical therapy, bracing, and in some cases surgery. Pain medications may also be helpful for individuals coping with unmanageable pain [3]. In some cases, foot drop due to muscular atrophy and slow nerve conduction can be managed by physical therapy [32]. Specialized shoes or orthoses can be extremely helpful with the management of foot drop and optimizing gait. Patients who are overweight or obese have also benefitted from additional weight loss and management [13] (Figure 1).
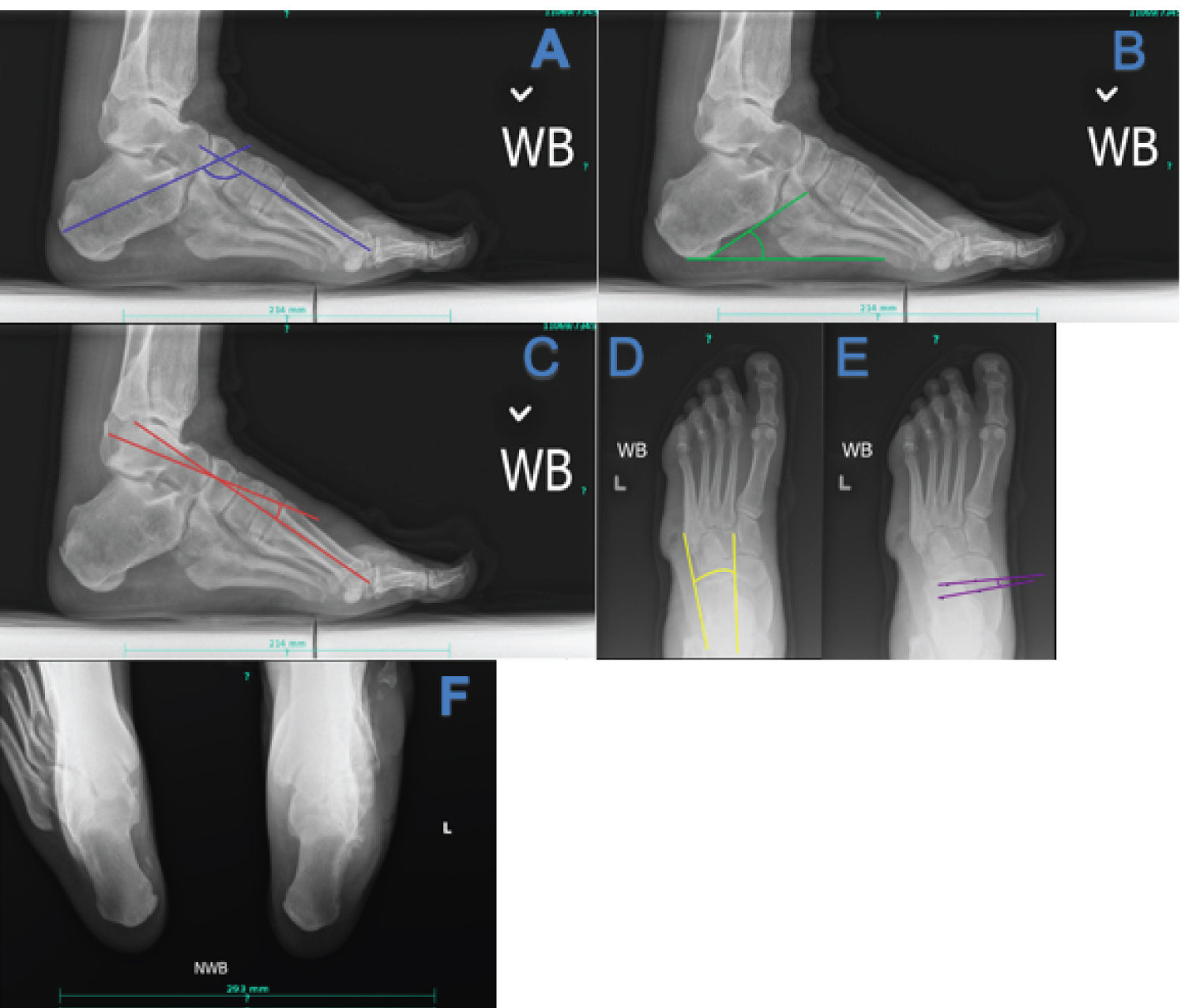 Figure 1: Key radiographic views and angles in CMT (a) Hibbs angle, decreased in cavus foot; (b) Calcaneal inclination angle, increased in cavus foot; (c) Lateral Meary's angle, increased in cavus foot; (d) AP talocalcaneal angle, decreased with hindfoot varus; (e) Talonavicular coverage angle, increased in cavus foot; (f) Long leg calcaneal axial view can be useful in determining the relationship of the calcaneus to the tibia. View Figure 1
Figure 1: Key radiographic views and angles in CMT (a) Hibbs angle, decreased in cavus foot; (b) Calcaneal inclination angle, increased in cavus foot; (c) Lateral Meary's angle, increased in cavus foot; (d) AP talocalcaneal angle, decreased with hindfoot varus; (e) Talonavicular coverage angle, increased in cavus foot; (f) Long leg calcaneal axial view can be useful in determining the relationship of the calcaneus to the tibia. View Figure 1
Should conservative treatment fail, surgical management may be considered. The goal in all treatment options is to provide a stable framework for gait and preservation of joint mobility when possible. The following treatment algorithm will be based on a pes cavus type foot structure, however it should be noted that each patient should be assessed individually and treatment tailored specifically to that patient, as other deformities including pes planus may be seen in CMT (Figure 2 and Figure 3) [33].
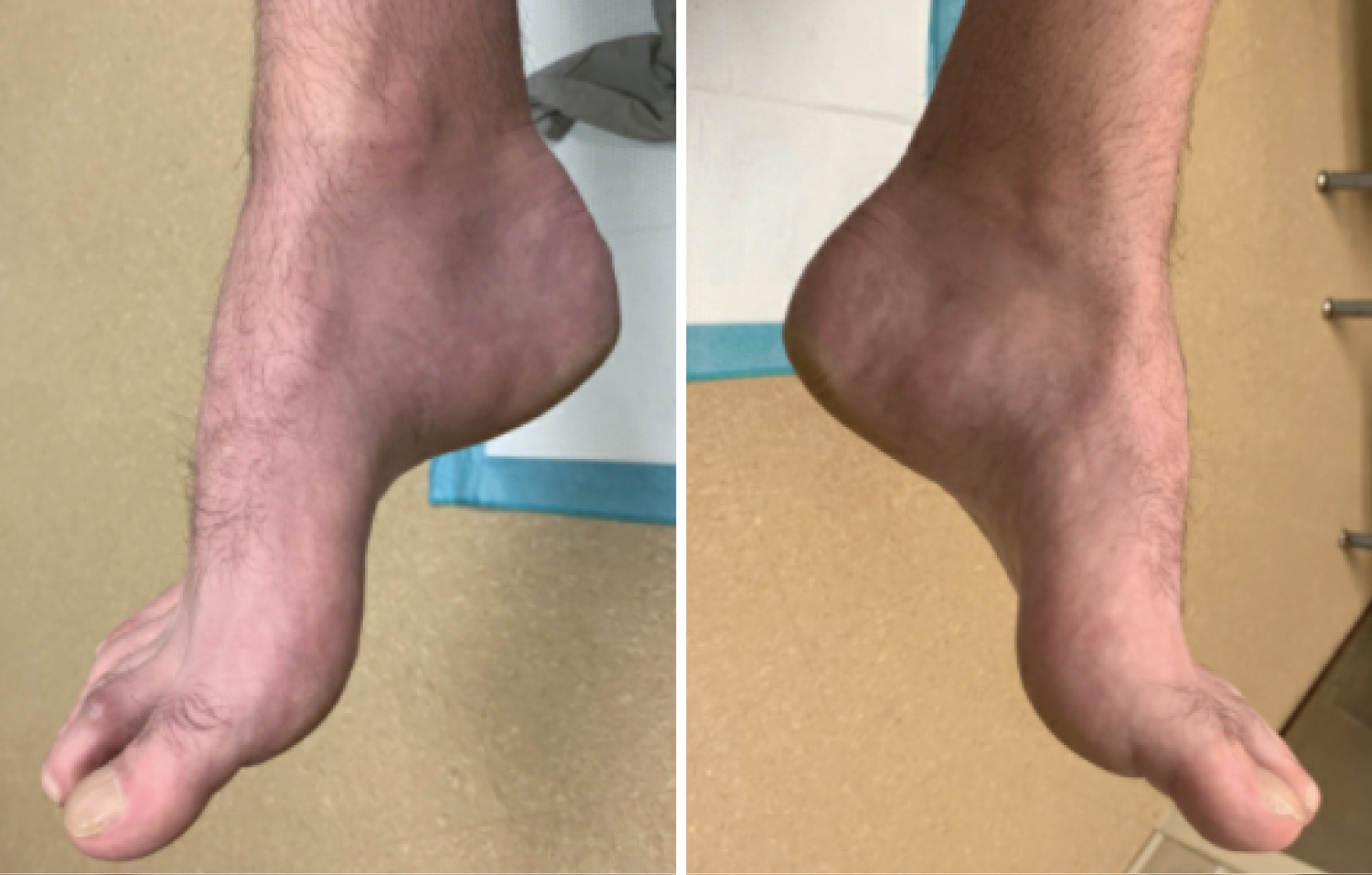 Figure 2: Non-weight bearing clinical images of right and left foot in a patient with CMT demonstrating cavus foot type. View Figure 2
Figure 2: Non-weight bearing clinical images of right and left foot in a patient with CMT demonstrating cavus foot type. View Figure 2
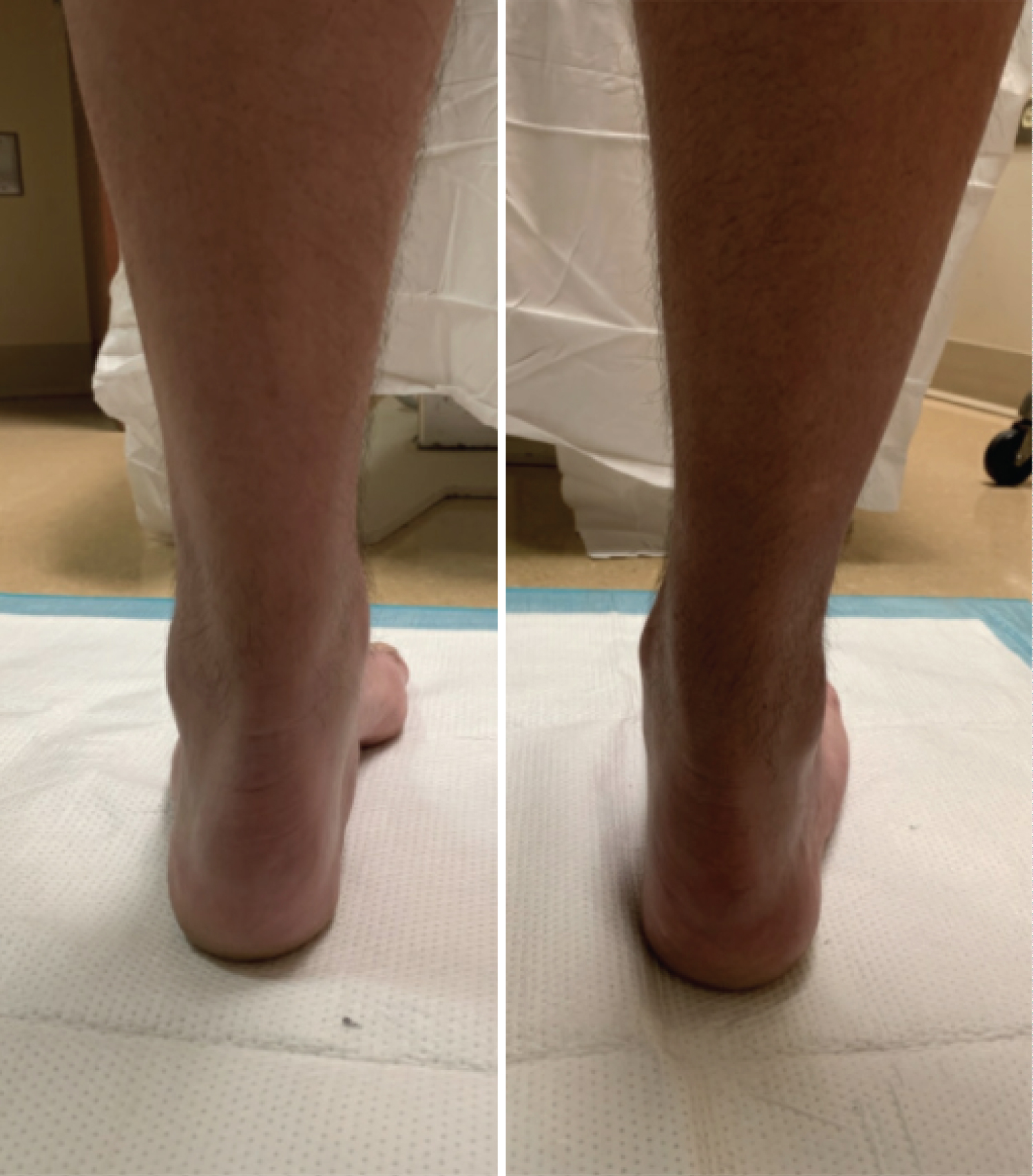 Figure 3: Weight bearing clinical images of left and right foot of patient with CMT. View Figure 3
Figure 3: Weight bearing clinical images of left and right foot of patient with CMT. View Figure 3
Often a predictable indication of imbalance within the foot are callosities, and these should help clue the practitioner in to what those imbalances may be. Assessment of the hindfoot position with the patient standing, as well as Coleman block testing is critical in determining fixed vs. reducible hindfoot deformity and whether varus deformity is forefoot or hindfoot driven. If the hindfoot corrects to neutral or valgus positioning, the hindfoot deformity is flexible and is derived from a plantar flexed first ray, and in most scenarios, hindfoot osteotomy may be avoided. Midfoot deformity is often missed on examination. With the hindfoot in a corrected position, the position and flexibility of the midfoot may be assessed. Fixed deformity may need to be corrected with a hindfoot osteotomy to achieve a plantigrade foot.
Furthermore, thorough manual muscle testing in the lower extremities is key, namely tibialis anterior, tibialis posterior, peroneus longus, and peroneus brevis, especially if tendon transfer is to be considered. Silfverskiold testing should be performed, however it should be noted that Beckman, et al. demonstrated that less than 25% of CMT patients within their cohort had a true gastrocnemius-soleal derived equinus [34].
Those with flexible deformity should be considered for tendon transfer and osteotomy and those with fixed deformity should be considered for joint fusion, osteotomy, and soft tissue rebalancing [34]. As with pes planus, those with advanced BMI or underlying joint arthrosis, procedure selection may need to be biased toward arthrodesis for optimal outcomes.
Medial Soft Tissue Release: Regardless of the extent of deformity, release of the medial soft tissues, namely the subtalar joint and talonavicular capsules should be performed. This will allow reduction of the coronal plane to make the foot plantigrade with additional procedures. Although rarely, sometimes a tarsal tunnel release is required if there is severe varus and inversion of the rearfoot.
Tibialis posterior transfer: The tibialis posterior muscle is often preserved in CMT and is a significant contributor to the varus deformity. Transfer to the dorsal aspect of the lateral cuneiform has a doubled benefit of decreasing the varus pull, but also helping aid in dorsiflexion of the foot. This may be performed by a standard 4 incision approach. This approach also helps to allow for intramuscular fractional lengthening of the long flexor tendons should clawing of the digits be a significant issue.
Bridle procedure: Originally described by Riordan and later modified, the Bridle procedure involves direct insertion of the Tibialis-Posterior into the middle cuneiform with tenodesis to the Tibialis Anterior tendon. The Peroneal Longus tendon is rerouted in front of the lateral malleolus in order to balance the foot in dorsiflexion. This is usually performed at surgeon preference instead of a traditional Tibialis Posterior transfer [35].
Plantar fascial release or steindler stripping: Often the plantar fascia becomes contracted in a cavovarus deformity. This may be released through a medial incision. Some surgeons include release of the flexor digitorum brevis and abductor hallucis attachments as part of the Steindler stripping procedure.
Peroneus longus to peroneus brevis transfer: This technique is used to correct a plantarflexed 1st ray if a dorsiflexory wedge osteotomy is not used. The peroneus longus function is often preserved in CMT, and transfer to the peroneus brevis has the added benefit of aiding with eversion. Additionally, if ankle range of motion is maintained, this can be performed in place of a tibialis-posterior transfer.
Lateral ligament reconstruction: Those with cavovarus deformity are predisposed to lateral ligament injury and upon correction of deformity may need augmentation. Anatomic repair or non-anatomic repair methods may be utilized, depending on the severity and the surgeon's preference.
Soft tissue equinus correction: This is rarely needed in this population as previously described, however more mild correction warrants gastrocnemius recession and more severe equinus warrants Tendo-Achilles lengthening. However, lengthening of the posterior muscle group can sometimes help reduce the varus pull which may be beneficial, although rarely performed.
Digital arthroplasty or arthrodesis: Debulking of the digital contracture can allow reduction of the toe. If soft tissue procedures are unable to allow complete reduction, sometimes fusion of the digital contracture is favored. Dorsiflexory Wedge Osteotomy of the First Metatarsal: This powerful procedure utilizes a closing dorsal wedge to correct plantarflexion of the 1st ray. May be utilized with or without accompanying lateralizing calcaneal osteotomy depending on the deformity.
Midfoot osteotomy: If more than two dorsiflexory wedge osteotomies are required in the midfoot (First and second metatarsal), a midfoot osteotomy may be preferred to correct the midfoot deformity. Care should be taken for the osteotomy to address all three planes of deformity.
Lateralizing calcaneal osteotomy or dwyer osteotomy: Utilized for hindfoot varus that cannot be fully reduced or in cases of rigid midfoot deformity in order to achieve a more plantigrade foot.
Supramalleolar osteotomy: If a weight bearing AP ankle radiograph demonstrates greater than 10 degrees of varus angulation, then a supramalleolar osteotomy may be considered. Either a medial opening approach with structural bone graft or closing lateral approach may be utilized. Limb length discrepancies should be considered here. Bilateral long-leg films may be useful in determining which procedure is indicated. Note that in severe cases of cavovarus deformity, derotational osteotomy may need to be performed to align the second ray with the tibial crest.
Triple Arthrodesis: In patients with a completely rigid deformity, significant degenerative arthrosis, or should they have severe obesity that would preclude them from joint sparing procedures, a subtalar, talonavicular, and calcaneocuboid arthrodesis may be warranted.
Ankle arthrodesis: This should rarely be considered within this cohort, as most with significant ankle arthrosis have accompanying hindfoot deformity, and thus pantalar arthrodesis be the only viable option.
The patient is a 52-year-old male police officer with a past medical history of Charcot-Marie-Tooth disease, current 1 pack per day smoker, history of left C5-7 anterior cervical discectomy with fusion several years ago, and peripheral neuropathy who presented for a second opinion of bilateral foot pain. He was diagnosed previously via EMG and NCV with CMT. Subjectively, the patient states that he progressively has developed severe cavus deformities to both of his feet and has had to modify his activity level due to pain and weakness. Denies any familial history of high arches or diagnosis of CMT. He has failed conservative treatment including orthotics, corticosteroid injections, as well as formal physical therapy. He takes gabapentin 1200 mg at night for his neuropathy. He states that the pain that he has in his feet feels different from his baseline neuropathic discomfort and describes it as more throbbing, aching, and stiffness.
On physical examination there was a lack of epicritic sensation to the feet bilaterally, generalized tenderness to both feet, this was most notable in the plantar arches along the prominent plantar medial band of the plantar fascia. Notable cavus foot type bilaterally with the left slightly worse than the right. Resting calcaneal stance revealed a varus attitude of the calcaneus on both feet, which was non-reducible with the Coleman block test. Manual muscle testing revealed weakness in DF, PF, inversion, eversion with 4/5 strength throughout, with notable mild peroneal muscle fatigue. Gait analysis demonstrated abnormal and rapid subtalar joint supination during initial heel strike and supinated position of the STJ throughout the stance phase of gait. There was no ankle instability, peroneal tendon pain, and no pain laterally on the calcaneus over the peroneal tubercle (Figures 2, Figure 3 and Figure 4: Clinical Images).
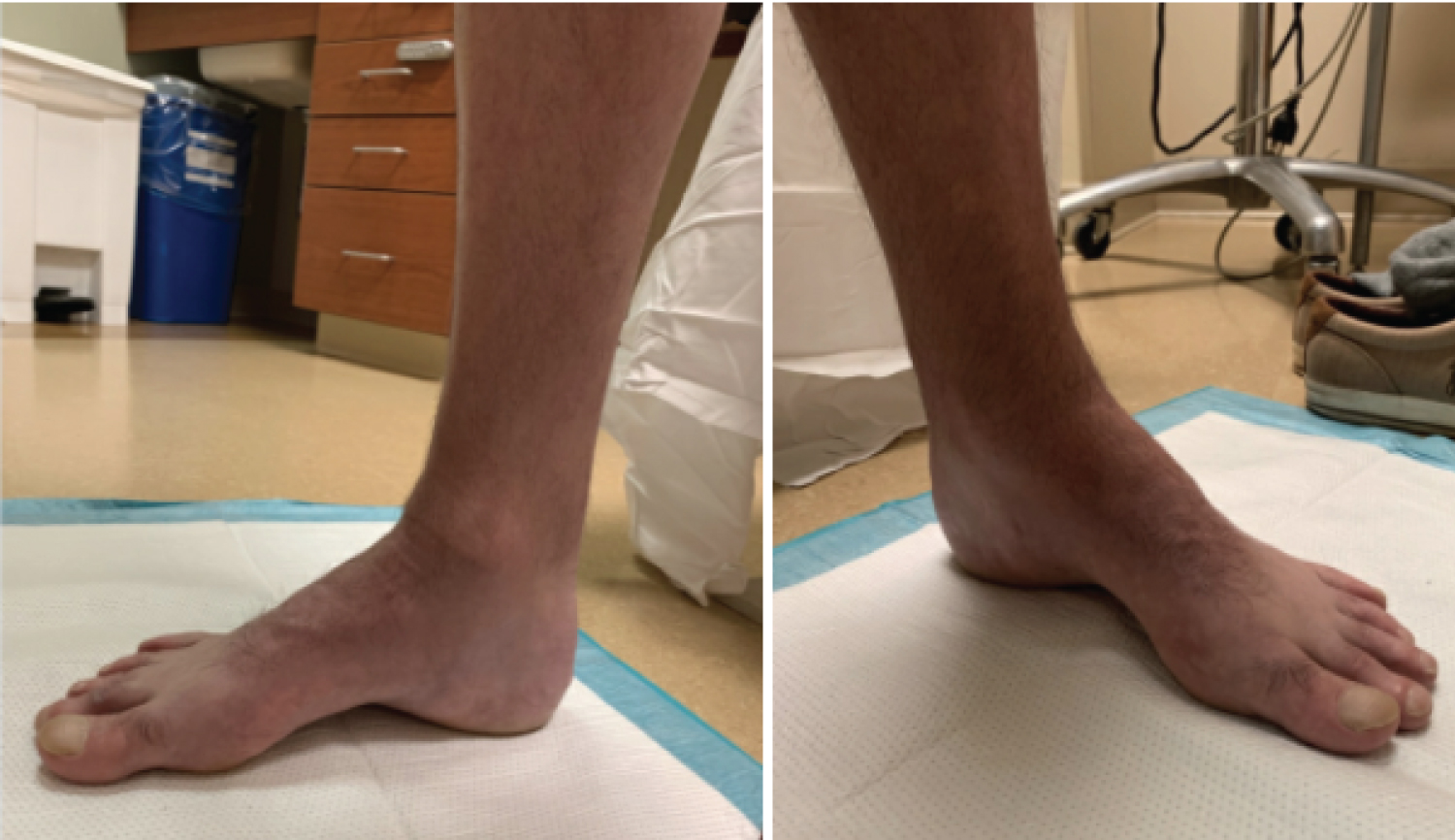 Figure 4: Weight bearing clinical images of a patient with CMT demonstrating a high longitudinal arch. View Figure 4
Figure 4: Weight bearing clinical images of a patient with CMT demonstrating a high longitudinal arch. View Figure 4
Standard views of the feet were obtained along with calcaneal axial view, most notable for varus attitude of the calcaneus, increased inclination of the calcaneus, increased lateral Meary's angle, and increased talar coverage (Figure 5). This all correlated clinically with the cavovarus deformity noted.
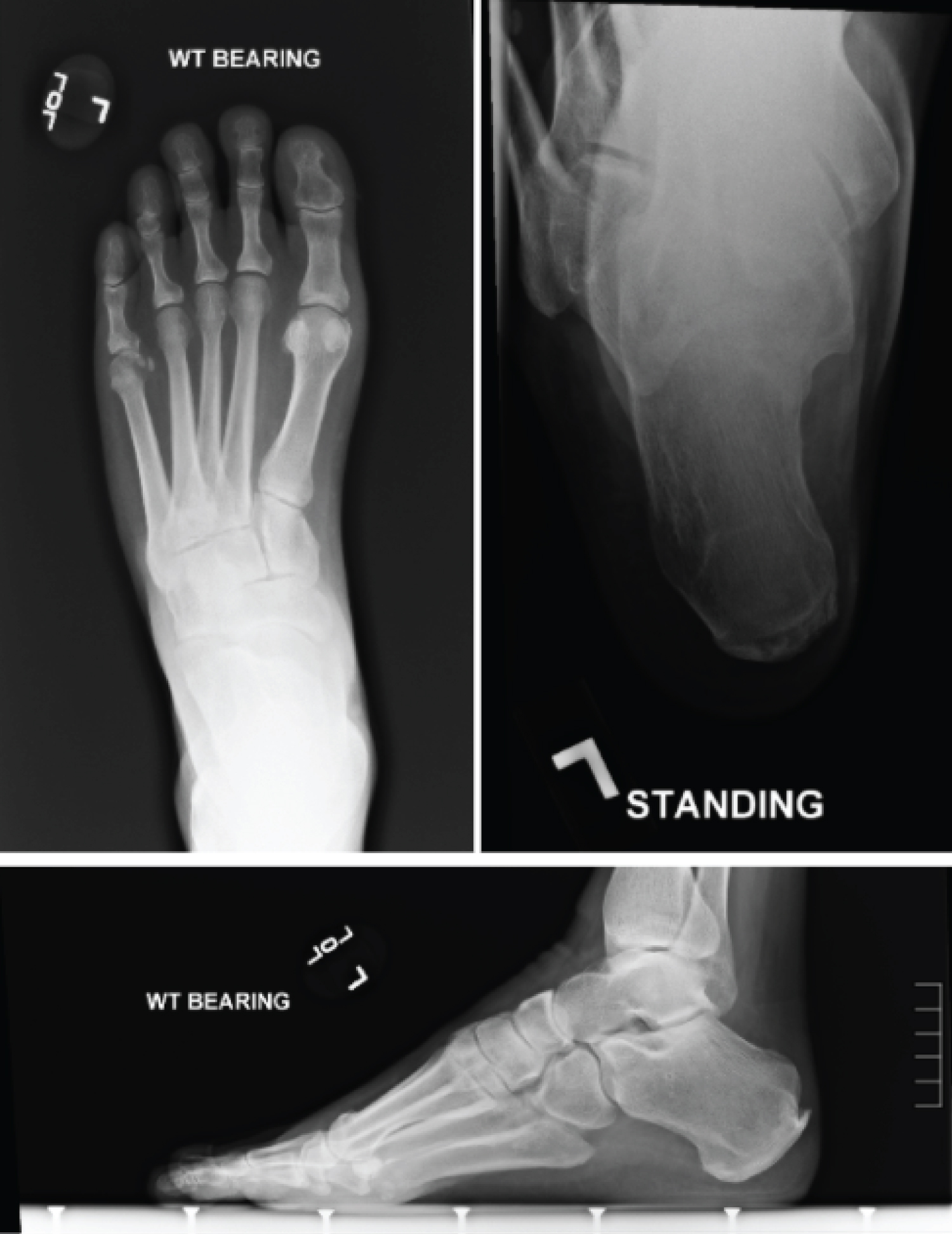 Figure 5: Radiographs of a patient with CMT. Top left: weight bearing AP view, top right: weight bearing calcaneal axial view, bottom: Weight bearing lateral view. View Figure 5
Figure 5: Radiographs of a patient with CMT. Top left: weight bearing AP view, top right: weight bearing calcaneal axial view, bottom: Weight bearing lateral view. View Figure 5
The patient was counseled on smoking cessation and vitamin D 25, OH levels were checked. Vitamin D 25, OH levels were deficient at 11.50 ng/mL (reference range 30.0-100.0 ng/mL) and he was started on oral cholecalciferol, 50,000 international units (IU) once weekly for 6 weeks. Computed tomography (CT) of the left lower extremity was ordered and did not reveal any significant arthrosis, cystic changes, or coalitions.
After medical optimization, the patient's left foot was surgically corrected with the following procedures, respectively: Steindler stripping, Dwyer, posterior tibial tendon transfer to lateral cuneiform, and dorsiflexory wedge osteotomy of the 1st metatarsal. The patient was placed into a posterior splint and was made non-weight bearing. The patient was transitioned to a CAM boot at the first postoperative visit, however remained non-weight bearing for a duration of 6 weeks. After postoperative week 6, radiographs of the foot were taken and demonstrated union of osteotomy sites (Figure 6). The patient was then started with gradual weight bearing, transition out of the CAM boot, and formal physical therapy.
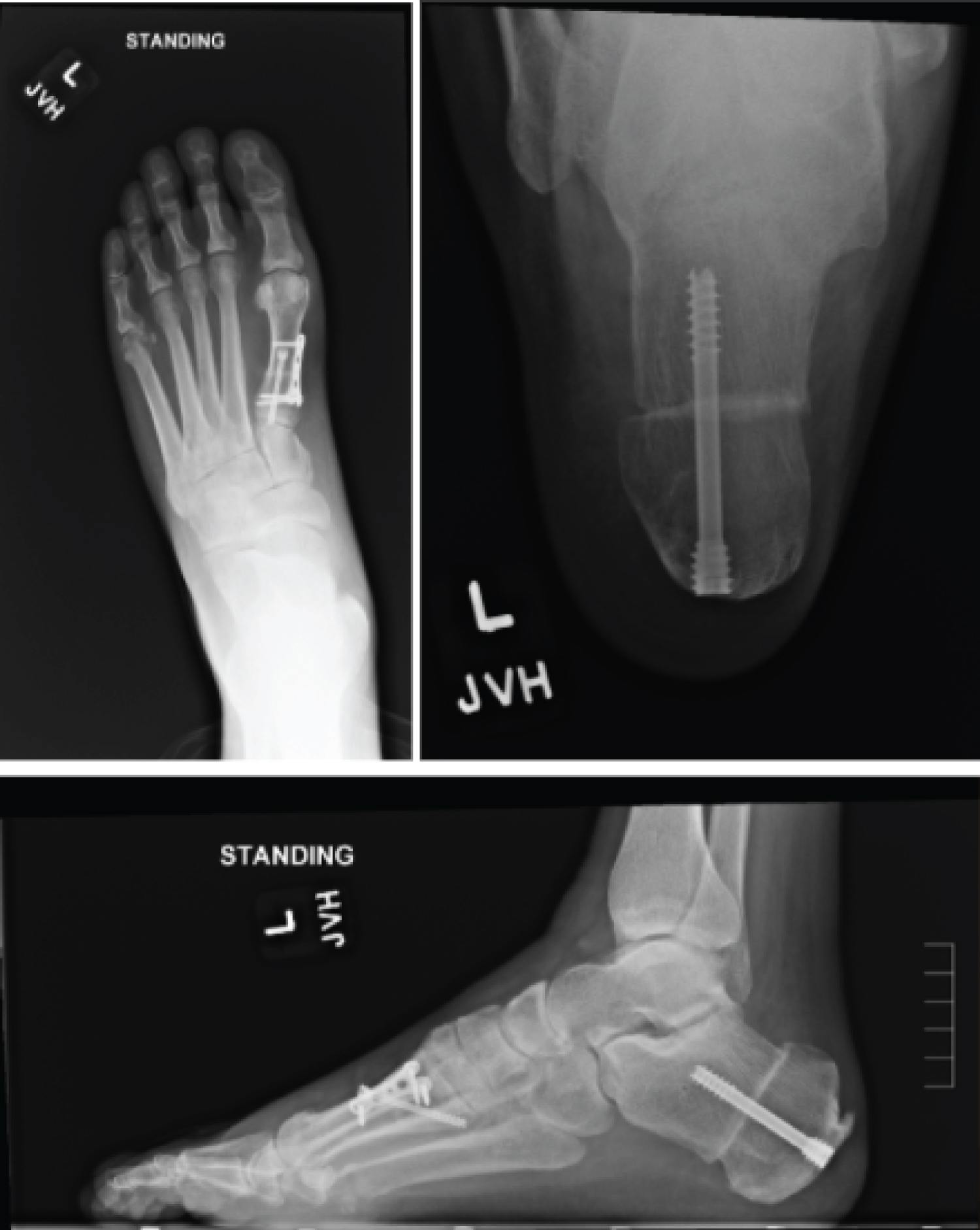 Figure 6: Postoperative radiographs of a patient with CMT who underwent reconstructive surgery. View Figure 6
Figure 6: Postoperative radiographs of a patient with CMT who underwent reconstructive surgery. View Figure 6
Now at two year follow up, the patient is doing well with no pedal complaints or functional limitations on the operative side and wishes to have the contralateral side surgically corrected (Figure 7).
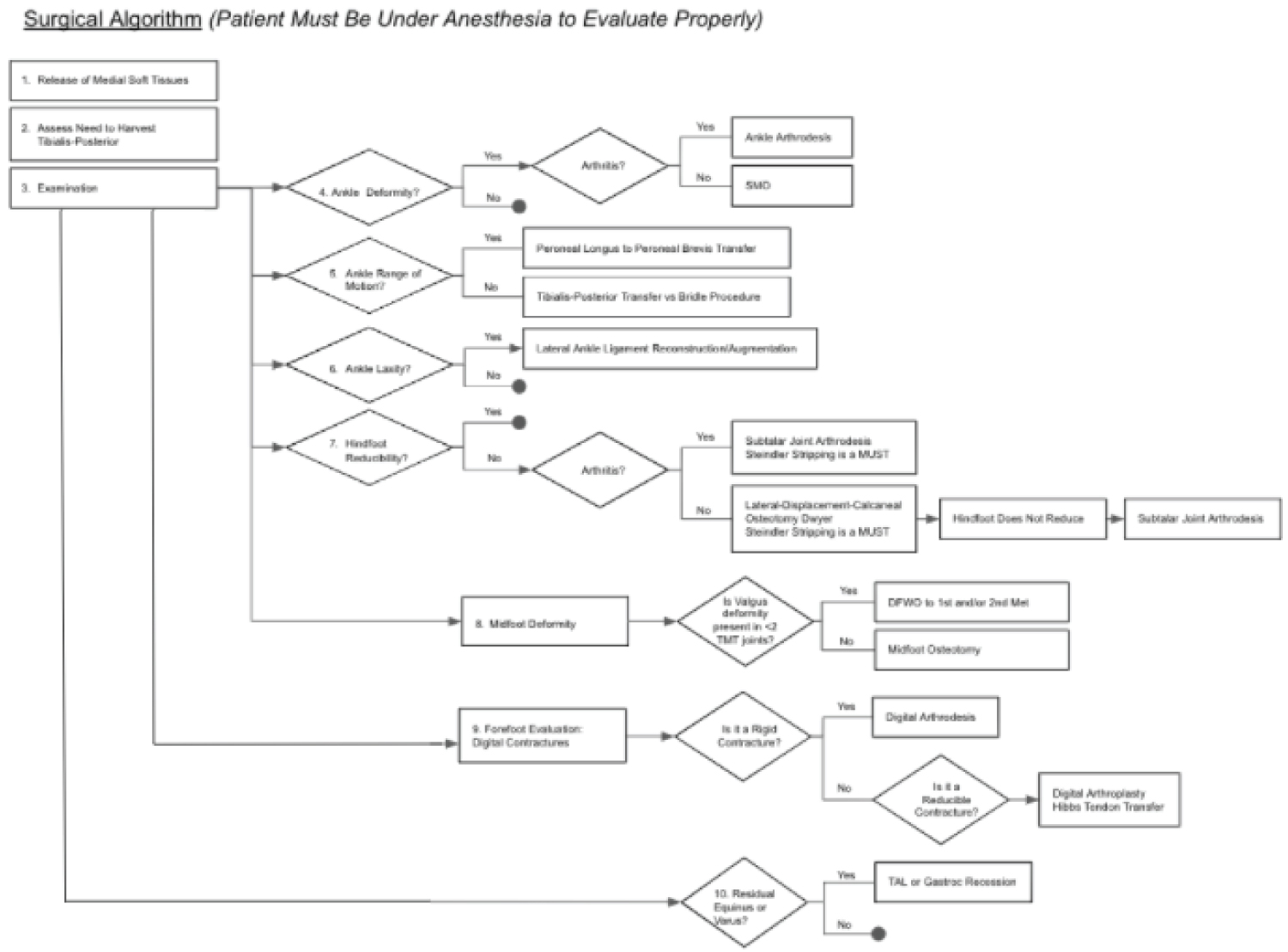 Figure 7: Stepwise algorithm for surgical reconstruction of the imbalances often seen in CMT. View Figure 7
Figure 7: Stepwise algorithm for surgical reconstruction of the imbalances often seen in CMT. View Figure 7
CMT is one of the most inherited neuromuscular disorders, typically with clinical symptoms of pes cavus, claw toes and foot drop, which greatly affects the gait cycle and overall quality of life in patients affected by these diseases. Difficulties developed in CMT are initially due to progressive weakness in distal muscles involving the lower extremities with later involvement of upper extremities [36]. The severity of symptoms increases with age and depending on the number of genes involved. EMG and nerve conduction therapies are used to categorize the disease and its progression. More research is needed in regard to genetic screening, diagnosis, prevention, and treatment of the various sequela to improve the quality of life for those with CMT [37-39].
Jonathan Furmanek, Amar Gulati, Anissa Hashemi-Awwal, Fahd M. Moeez, Prakash Panchani, Caitlin Zarick, Eugene Batelli, and Paul Carroll have no financial disclosures, commercial associations, or any other conditions posing a conflict of interest to report.
All authors declare there are no conflicts of interest.
AH conceived the study design, drafted the manuscript, and aided in obtaining the information. FM aided in drafting the manuscript and obtaining the information. PP aided in drafting the manuscript and in obtaining the information. All authors read and approved the final manuscript.