Every year ischemic stroke takes many lives and leaves millions of people with neurological deficits. Currently the only approved therapy is recombinant tissue plasminogen activator, which should be administered within a narrow time window of 4.5 hours. Stem cell therapy was first initiated in several preclinical studies with promising results and lately in some clinical trials. Our research consists of 2 systematic reviews where preclinical and clinical studies were pooled. We provide a systemic review of the evidence of efficacy of cell-based therapy in both preclinical and clinical setting.
After screening of databases, 76 studies were included in our systematic review of studies in rodent stroke models and 4 randomized clinical trials were used for the systematic review of studies in humans. After data extraction and assessment of study quality, the pooled effects were calculated using Revman5.
Stem cell therapy has a positive effect on behavior and histological outcome in rodent stroke models. These results are in line with previously conducted meta-analyses. This improvement in rodents is not translated into clinical trials in humans. Pooled study data of the randomized controlled clinical trials did show a significant improvement in neurologic outcome, but not in functional recovery.
Study quality is pointed out as one of the major reasons for failed translation of preclinical evidence to clinic. Large, well-designed preclinical trials are urgently needed. Good preclinical research is necessary to determine the optimal route of administration, the optimal cell dose and type and the most accurate administration time of the stem cells.
Systematic review, Stroke, Stem cells therapy, Translation, Human, Rodents, Neurorestoration
Stroke remains a major universal health problem. On yearly basis numbers of stroke patients who live with the consequences or subsequently died from it, are increasing. The ischemic stroke or infarction represents almost 70 percent of all strokes [1]. It is the second leading cause of global death [2].
Notwithstanding the advances in acute care and secondary preventive approaches, a stroke continues to be a major burden on the healthcare system worldwide. Currently the only approved medical therapy for patients with an acute ischemic stroke is recombinant tissue plasminogen activator (rtPA). International guidelines advise rtPA as a first-line treatment for eligible patients when administered within 4.5 hours after the onset of stroke [3,4]. Despite this recommendation, rtPA is widely underused [4]. Only a minority of patients receives thrombolysis, primarily because of delayed admission to a stroke center and the short time window for the use of rtPA [4]. Even when patients are fortunate enough to receive rtPA in the time frame, several neurological deficits persist [5]. After three hours neurological cells become irreversibly damaged [6]. Once stroke-induced cell damage has occurred, little can be done to improve the neurological function, except for rehabilitation therapy and pharmacological management of comorbidities [7].
It becomes clear that other approaches promoting recovery of neurological cells rather than neuroprotection need to be explored and developed. Observations from animal studies suggest that improved functioning is associated with restorative processes [5]. Following a stroke, patients show some recovery trough plasticity and brain remodeling, and questions are raised if cellular therapy could be used to tap into these recovery processes and promote recovery of function [5]. The Stroke Progress review group has now identified neurorestoration as a major priority for stroke research [8]. Cell therapies account for a different biopharmaceutical approach than rtPA. Whereas rtPA only has a protective role and a narrow efficacy time window, cellular therapies have more neurorestorative mechanisms of action. Stem cell transplantation is highly convergent with rehabilitation and has a wider therapeutic time window [9].
Cellular therapies comprise several cell types and different sources have been used in preclinical animal studies. These cell sources can be divided in three major groups: Embryonic or fetal cells, birth related cells and adult cells [9]. Although some cell types are more widely used in preclinical settings, there is still not enough evidence to withhold one particular cell type for clinical practice [8]. Several delivery routes can be used to administer these stem cells. Each delivery method has its safety issues, strengths and weaknesses. The stereotactic, intracerebral administration of stem cells relies on the tissue restoration hypothesis [10]. This delivery route has the advantage that more cells are implanted in the lesion site, but it may cause several safety issues and concomitantly brain secondary damage [8,10]. Intra-arterial administration of stem cells happens by an injection close to target which enhances the homing to the brain [11]. Following the chemoattractant gradient generated by the ischemic brain some of the stem cells penetrate the blood brain barrier. The number of cells reaching the lesion site is smaller than with intracerebral administration, but grafted cells do not necessarily have to be near the lesion site to have effect [8]. Intra-arterial delivery is an easy administration route with minimal invasiveness. It leads to a widespread cell distribution and secretion [8]. There is a lower risk of cell trapping, but large amounts of cells pose the hazard of secondary infarctions by microvascular plugging in the brain [9-11]. Intravenous administered cells do not reach the infarcted brain region in sufficient quantities to have a direct neurorestorative effect [9]. The cultured cells are indeed too big to pass the capillaries of the filtering systemic organs and are trapped in the lungs and spleen [10]. Cell homing is thus comprised, but intravenously administered stem cells do impact brain inflammation and repair through bystander mechanisms [8,9]. Intravenous injection is also considered as a safe, minimal invasive delivery route with wide distribution of cells [11]. The trapping in lungs and spleen however, poses the risk of splenic and pulmonary infarction [10,11]. Intracerebral administration is considered too invasive to be used in clinical practice, so intravascular injection definitely seems a more useable method [12]. Unfortunately, there are no valid studies directly comparing the different vascular cell routes. Hence, it is too early to make clear statements about the optimal time, dose and cell delivery route. Route of administration thus poses another problem in the design of clinical trials [8].
Several studies in animal models of ischemic stroke have shown that stem cell transplantation can lead to structural and functional improvement [13,14]. The evidence for benefits in ischemic stroke patients however, is still lacking. In 2010 the Cochrane collaboration tried to establish a meta-analysis of randomized controlled trials (RCT) in humans. But with only one valid reference it became clear that more studies are desperately needed [7]. This review consists of 2 systematic reviews and provides a comparison between preclinical animal studies and clinical studies in humans about the use of stem cells as a therapy for a stroke.
Animal studies of stem cell therapies for stroke were identified from electronic databases PUBMED and EMBASE. The following search strategy was used to screen PUBMED: (((((((cell transplantation) OR stem cell transplantation) OR cord blood stem cell transplantation) OR hematopoietic stem cell transplantation) OR mesenchymal stem cell transplantation) OR peripheral blood stem cell transplantation)) OR ((((((((((((((stem cells) OR adult stem cells) OR multipotent stem cells) OR totipotent stem cells) OR pluripotent stem cells) OR mesenchymal stem cells) OR fetal stem cells) OR embryonic stem cells) OR hematopoietic stem cells) OR tumor stem cells) OR myeloid progenitor cells) OR fibroblast))) AND (((((((((((cerebrovascular disorders) OR basal ganglia cerebrovascular disease) OR brain ischemia) OR stroke) OR middle cerebral artery) OR MCA) OR anterior cerebral artery) OR ACA)))) AND ((((Rodent) OR Rat) OR mouse)) AND (((((controlled) OR control group) OR sham) OR placebo) OR comparison). The number of references were narrowed by using the filters 'Other Animals' and 'English'. This research strategy was used to screen EMBASE as well. Duplicates excluded, a total of 780 references were imported in Endnote for further screening. 514 references were excluded (Figure 1). The remaining 266 studies were subject to full reading of abstract and quick view on the full text. 97 studies were isolated for intensive reading of full text. Eligible studies were vehicle-controlled studies that reported histological (infarct size) or behavioural outcome of allogeneic or autologous stem cells, stem-like or progenitor-like cells in adult rodent models of focal cerebral ischemia. The ischemic stroke had to be induced trough transient or permanent anterior or middle cerebral artery occlusion. Studies were excluded if the therapy involved additional active components such as bioscaffolding or gene modification other than labelling or tracing markers or if the studies involved co-transplantation of different stem cells. Finally, 76 references, with 101 treatment arms fulfilled all proposed criteria and were included in the systematic review.
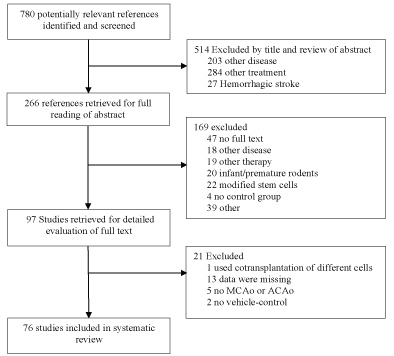 Figure 1: Flow diagram of the preclinical studies.
View Figure 1
Figure 1: Flow diagram of the preclinical studies.
View Figure 1
Studies of stem cell therapies for stroke in humans were identified from electronic databases PUBMED, EMBASE and the Cochrane Central Register of Controlled Trials (CENTRAL). The following search strategy was used to screen PUBMED: (((((((cell transplantation) OR stem cell transplantation) OR cord blood stem cell transplantation) OR hematopoietic stem cell transplantation) OR mesenchymal stem cell transplantation) OR peripheral blood stem cell transplantation)) OR ((((((((((((((stem cells) OR adult stem cells) OR multipotent stem cells) OR totipotent stem cells) OR pluripotent stem cells) OR mesenchymal stem cells) OR fetal stem cells) OR embryonic stem cells) OR hematopoietic stem cells) OR tumor stem cells) OR myeloid progenitor cells) OR fibroblast))) AND ((((((((cerebrovascular disorders) OR basal ganglia cerebrovascular disease) OR brain ischemia) OR stroke) OR middle cerebral artery) OR MCA) OR anterior cerebral artery) OR ACA). This search strategy was modified to search the other databases. In PUBMED the filters 'humans' and 'clinical trial' were activated. In EMBASE the search was limited to 'humans' only. Duplicates excluded, a total of 806 references was found. 532 were excluded. The remaining 274 studies were subject to full reading of abstract and quick view of the full text. 16 articles were isolated for reading of full text. Finally, 4 randomized controlled clinical trials (RCTs) were used in the systematic review (Figure 2). All the RCTs involved patients with ischemic stroke, a minimum of six-month follow-up and efficacy as primary or secondary outcome.
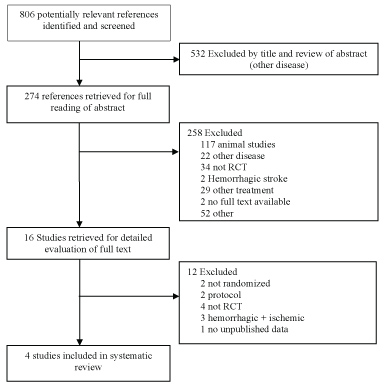 Figure 2: Flow diagram of the clinical studies.
View Figure 2
Figure 2: Flow diagram of the clinical studies.
View Figure 2
We withdrew data from all available sources in each article, including text and graph. When solely graphic presentation was accessible, values for mean and standard deviation (SD) were manually obtained. When outcomes were reported at numerous time points, only the final assessments were accepted. We used RevMan version 5.3 [15] for all data and analysis. A P-value of < 0.05 was considered significant.
Efficacy of stem cell transplantation in animal models was assessed trough two outcomes: Behavior and histology. These outcomes were analyzed separately and so when both were reported, we extracted both. Our statistical methods were based on previous meta-analyses [13,14].
For the assessment of the effect on behavior, the nine most frequently applied behavioral tests were incorporated in the analysis: Modified neurologic severity score (mNSS), cylinder test, rotarod test, beam walk, treadmill stress test, forelimb reaching, body swing, corner test and adhesive removal test. Infarct volume was used as the representation of the histologic outcome. All functional and histologic data were inserted in RevMan as continuous variables with mean and standard deviation. If the standard error (SE) or 95% confidence interval (95% CI) was given, the standard deviation was calculated using these data before entered in RevMan. The inverse-variance method was used as a statistical method to examine the mean effect size, 95% confidence intervals, forest plots, and significance. The effect measure was set as standard mean difference because infarct volume was measured in a variety of ways [15]. Substantial heterogeneity was present across the functional (I2 = 82%) and the histologic endpoint [I2 = 72%], so random effects models were used as analysis model.
To estimate the methodologic quality for each preclinical study the Quality score for methodologic quality of Lees, et al. which defines 10 criteria based on STAIR guidelines was used [13,14]. The checklist comprises 10 criteria by which methodologic quality can be assessed. For the calculation of a mean study quality score one point was attributed for each criterion reported. The scores range from 0 to 10, with higher scores indicating greater methodologic accuracy [13,14].
To evaluate the efficacy of stem cell transplantation on functional and neurological outcome in clinical setting, we incorporated the three most frequently used tests [the modified Rankin scale (mRS) and Barthel index (BI) for functional evaluation and the National Institute of Health stroke scale (NIHSS) score as neurological outcome]. Data were administered as continuous variables with mean and SD. If necessary, the SD was calculated using the 95% CI. The mean effect size, 95% CI, forest plots and significance of stem cell therapy on functional and neurological outcomes were calculated in Revman5 using inverse-variance method. The effect measure was set as mean difference. Substantial heterogeneity occurred, so random effects were set as analysis model.
We evaluated the risk of bias in each study by verifying the presence of allocation concealment, outcome blinding, intention-to-treat analysis and addressment of incomplete outcome data (losses to follow-up) [7].
In total 76 publications comprising 101 stem cell treatment arms were included (Table 1). 94 of these treatment arms reported behavioral outcomes trough a total of 134 results of 9 different behavioral tests. Histologic outcome was reported by 69 of the 101 treatment arms. Infarct volume was described in either absolute or relative terms. The absolute lesion volume was given as mm3, while the relative was given as a percentage compared to the volume of contralateral (non-infarcted) brain hemisphere or of the total brain volume.
Table 1: Study characteristics of preclinical studies. View Table 1
The median (Q1, Q3) quality score of the included studies was 4 (3-5), with a range from 1 to 7. Sample size calculation, allocation concealment and the use of animals with relevant comorbidities were the quality criteria that were least addressed in the incorporated studies. The proportions of studies meeting each Quality Score criterion is given in Table 2. There was also a substantial degree of heterogeneity for the histologic outcome analyses: I2 = 72%.
Table 2: Quality score of preclinical studies. View Table 2
The overall effect size, that is the improvement in functional outcome for stem cell-treated rodents relative to the control group, is 1.83 (Figure 3). Cell therapy thus has a positive effect on the behavior of post-stroke animals. Concerning the infarct size, the overall effect size for cell-treated animals is -1.17 (Figure 4). These values represent a significant effect. When assessing the infarct size, it is important to correct for brain edema, as this can result in an overestimation of lesion volume. The swelling of the brain may vary in degree from brain to brain and can introduce major defaults in volume determination [16]. We can correct for brain edema by using the indirect measure method [16,17]. The corrected effect size is -0.95 (P < 0.00001), which still represents a substantial and significant improvement.
 Figure 3: Forest plot of functional outcome.
View Figure 3
Figure 3: Forest plot of functional outcome.
View Figure 3
 Figure 4: Forest plot of structural outcome.
View Figure 4
Figure 4: Forest plot of structural outcome.
View Figure 4
The 4 RCTs all used autologous cell transplants in severe or moderately severe models of ischemic stroke. No sham control of bone marrow aspiration was performed in the control groups. All transplant and control groups received conventional treatment. One study excluded patients that had received rtPA [18]. All studies had a long-term follow-up of at least 1 year.
We evaluate the methodological quality of the clinical trials (Table 3) using criteria based on the Cochrane criteria [7]. In each study, allocation concealment, blinding of outcome evaluators, analysis by intention- to-treat and losses to follow-up were investigated. The heterogeneity of incorporated data was assessed using the I2-test. A considerable degree of heterogeneity was seen in all the included studies (Figure 5, Figure 6 and Figure 7).
Table 3: Methodological quality of included RCTs. View Table 3
 Figure 5: Forest plot of Barthel index.
View Figure 5
Figure 5: Forest plot of Barthel index.
View Figure 5
 Figure 6: Forest plot of Modified ranking scale.
View Figure 6
Figure 6: Forest plot of Modified ranking scale.
View Figure 6
 Figure 7: Forest plot of National Institute of Health Stroke scale.
View Figure 7
Figure 7: Forest plot of National Institute of Health Stroke scale.
View Figure 7
The BI score, with 138 included patients, and the mRS, with 220 included patients, showed no significant difference between the stem cell transplanted group and the control group. The NIHSS, with 109 included patients, did show a significant improvement in neurological function in the transplanted group (P = 0.03) (Figure 5, Figure 6 and Figure 7).
Preclinical studies of stem cell therapy for ischemic stroke in rodent models displays an overall significant improvement in behavior and histology after stem cell transplantation. The preclinical pooled data analyses all established large beneficial effect sizes. In comparison with previously performed meta-analyses, the trend in our result was similar to the published data [13,14,19].
Based on the evidence gathered from preclinical studies, several clinical trials were designed. To our knowledge, this is the first systematic review of randomized controlled trials on the use of stem cell therapy in clinical stroke models. Even though the fact that preclinical-pooled data analyses all established large beneficial effect sizes, stem cell therapy does not significantly improve the functional outcomes of patients with ischemic stroke. Neurological outcome did show a significant improvement, but only 2 studies calculated the NIHSS, which makes this the least prevailed factor. We can conclude that there is a trend for improvement by cell-based therapy, but currently this does not lead to a significant result.
We found 2 other non-randomized clinical trials with comparable baseline characteristics that reported efficacy of stem cell therapy in clinical setting [20,21]. The first study confirmed our results as it reported that no significant differences in neurological function were found [20]. The second study showed a statistically significant improvement in the modified BI in the transplanted group. However, due to a small sample size, to non-randomization and an unblinded outcome assessment in this study, make it less convincing [21].
Despite several attempts, the documented efficacy in preclinical rodent studies has not yet been translated to successive clinical trials. The study qualities or the therapeutic modalities could explain this failure.
The mean quality score for the included preclinical studies was only 4/10. Quality of preclinical studies was also reviewed in other meta-analyses and this shortcoming was highlighted [13,14,19]. This deficiency raises the consideration that authors and/or the reviewers did not adequately identify the potential limitations of the experimental design and/or data analysis. Lacks sample size and allocation concealment were two reported criteria of the poor-quality score. The absence of both sample size calculation and allocation concealment can cause an overestimation of the treatment effects. Low methodological quality reduces the benefit of the obtained results, since several studies have indicated that there is a relationship between increasing study quality and declining efficacy of stem cell therapy. Inadequate reporting thus correlates with overestimated efficacy [14,22,23]. However, some contrasting information is gained in a recent meta-analysis where higher study quality score was associated with larger behavioral gains related to mesenchymal stem cell administration [13].
The considerable degree of heterogeneity is an affirmation of the lack in standardization in the administration and reporting of experiments. The stroke research community recognized these inadequate study designs as one of the major reasons for the failed translation of positive results from animal studies to clinical trials [23]. The quality of preclinical studies has an important relevance in the translational potential of preclinical results. To overcome these barriers in translation, a conference of academicians and industry representatives was gathered to recommend guidelines for the preclinical improvement of quality of acute ischemic stroke therapies [24]. This meeting led to the first Stroke Therapy Academic Industry Roundtable (STAIR) publication in 1999, which was updated in 2009 [22]. The STAIR group reconciled in 2001 as well, but then to discuss various aspects of the design of clinical trials for stem cell therapy for stroke. This is equally important in the translational process, as the chances for future success trials in clinical setting [25].
Despite the initial guidelines, many questions and unresolved issues remained, so in 2007 another meeting with investigators from academia, industry leaders, and members of the National Institutes of Health was held. This Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS) meeting established on the roadmap of the STAIR meeting and explored the remaining concerns with the intention of better understanding of the status of the field. Their goal was to create a plan for future laboratory and clinical investigations in order to accelerate the process towards an effective cellular therapy for stroke [26].
After the publication of the original STEPS guidelines in 2009, an enormous growth in the number of cellular products and new laboratory experimentations occurred. The newly generated information had an impact on the safety and efficacy of cell-based therapies for stroke, so in 2010, a second meeting called STEPS 2 was convened. The purpose of this meeting was to identify critical gaps in knowledge and research areas, as well as update the prior recommendations and formulate new guidelines to direct future investigations [27]. Although meetings such as STAIR and STEPS integrate free debate between important investigators in the field and have the intention to improve (pre)clinical trials by this interchange, application of the STAIR model seemed too difficult to achieve and the first STEPS meetings did recognize these issues, but without offering specific solutions [28].
In 2012, a workshop on Improving the Quality of Preclinical and Clinical Research through Rigorous Study Design and Transparent Reporting (RIGOR) was held. The objective of the workshop was to draft guidelines to ensure worldwide consistency in stroke research practices. The RIGOR guidelines should be incorporated into translational grant applications and are recommended for all manuscripts submitted to Translational Stroke Research [29].
In December 2011, investigators from academia, industry leaders and members of the National Institutes of Health and FDA gathered again at the third STEPS meeting, STEPS 3. They discussed emerging data on the mechanisms of action of cell therapy, the barriers to successful clinical translation of preclinical evidence and the design of current clinical trials for acute and chronic stroke. Since the prior STEPS meetings, the field has continued to progress, and pilot clinical studies started to show safety for some cell types. It became clear that the formulation of a new set of guidelines, which focus on topics not covered in previous STEPS publications, was necessary. STEPS 3 displays a collection of recommendations that focus on more advanced stages of clinical testing [phase II/III], as well as testing of cell therapies in a broader stroke population like chronic stroke [30].
Many similarities exist in the guidelines advised by STAIR, STEPS, and RIGOR. They all strive for adherence to good laboratory (GLP) practices, including the need for several animal modeling studies with blinding to condition and outcome, randomization and complete power analysis and statistical analysis. They also request the clear statement of these GLP when submitting translational grant applications and manuscripts [31].
A positive note to this whole issue is that study quality has improved over time. There is a statistically significant correlation between quality score and year of publication [19]. This result may be suggestive of better incorporation of and adherence to the quality standards over time. Although there might be some improvement, there is still a great lack of consistency in the field which makes it difficult to compare studies and truly ascertain optimal conditions. Standardization of outcomes must be an important focus for experts in the field [19,32].
The problem could also lie in the methodology of the clinical studies; however, we would expect that study bias would favor the transplanted group. The transplanted group did not do significantly better, so lack of methodological quality in clinical studies is not likely a reason for failed translation to clinic. Nevertheless, study quality is of major importance to make accurate conclusions. Several of the included RCTs reported lack of blinding of patients and due to ethical considerations, no sham or cell aspiration could be performed in the control groups. One RCT had no allocation concealment and 2 RCTs did not use an intention-to-treat analysis. Because of the small sample sizes, the clinical trials have limited power. Two of the studies did not report missing data. All these methodological inaccuracies are important and should be addressed in future clinical trials.
Besides study quality, equally essential in this quest for translation to clinic, is to find the optimal in vivo animal model and to determine the administration conditions with maximum efficacy.
Questions could be raised if the uses of rodent models for stroke for preclinical testing are the reason for the failed translation to the clinic. By other means, are rodents the correct animal model for preclinical testing trials? To translate stem cell therapy from the laboratory to the clinic in a successful manner, the resulting pathophysiological phenomenon of each stroke model should mimic the human disease condition as closely as possible. The anatomy of non-human primates (NHP) most closely resembles the human brain; besides their similar vascular anatomy there are numerous reasons to support the use of NPH models. NHP are gyrencephalic, researchers could target the basal ganglia through occlusion and their size makes it possible to test them by using human clinical techniques such as: CT, MRI, angiography, etc. NHP models are the best models to assess the cerebral ischemia pathology but, they require laborious post-operative care and are costly. This makes it difficult to accomplish in a large sample size, so safety rather than efficacy may be the more appropriate outcome measure [19,31].
Despite the benefits of NHP models, rodents have been mainly used for testing therapeutic windows in acute, subacute and chronic stages of stroke and many mechanisms of cell therapy on stroke were clarified by the use of these rat models [13,31]. The use of rodents does also have many advantages like ease in post-operative care, feasibility, the possibility of large sample sizes and low costs. Like humans, rats have an internal carotid artery that branches off into anterior and middle cerebral arteries, but unlike human, they display variability in the vascular supply [19,31]. Research also concluded that mouse models are not sufficient to study human inflammatory diseases [33]. Nevertheless, the logistical and practical advantages of rodents' stroke models overcome these technical limitations; present animal models for stroke are assigned to rodents even though this preclinical model does not completely reflect the human stroke model [13].
Most focal ischemic stroke models in rodents are produced by ligation or occlusion of the proximal or distal middle cerebral artery (MCA) by the insertion of sutures, photothrombosis or infusion of blood clots using various methods. Ligation of the middle cerebral artery is the most popular, but it does have limitations, including the high rate of variability with the location and size of the lesion [31]. Although these models can mimic both complete and transient occlusion, none of them accurately mimic a clinical stroke. Because in humans who do suffer a stroke, complete occlusion rarely occurs and there is usually a spontaneous recanalization. Furthermore, the context in which the animal stroke model is assembled differs in the circumstances found in a clinical stroke. At first, stroke patients vary in the most relevant characteristics and represent a very heterogenic group [33,34]. Not only do patients differ in premorbid neurological function, age and site of ischemic lesion, a range of other clinical considerations, like multiple stroke risk factors and concomitant diseases are also often present. These factors do not only play a critical role in the resulting pathology of stroke, they also affect the response to therapeutics and the recovery after stroke [31,34].
The problem is that the present preclinical models mostly evaluate the effect of stem cell therapy in homogenous, adult, healthy male animals and co-morbidity factors and complications are not addressed. As a reaction on this, the STAIR publications emphasized that inclusion of comorbidities such as hypertension, diabetes and hypercholesterolemia in preclinical investigations is necessary for the successful translation of stem cell therapy for ischemic stroke. Female and aged animals should be included to generate a more heterogenic group of rodents so that animal models mimic human disease as closely as possible [19,22,29]. The previous recommendations were based on the idea that studies in animal models with comorbidities would better replicate the pathophysiological state of human stroke patients. So, to effectively translate preclinical models to clinic, the use of appropriate animal models that mimic human diseases were seen as mandatory [29,31]. However, this hypothesis is challenged by the fact that human strokes occur in a mixed gender aged population in which most people have a history of hypertension or diabetes, but these are usually controlled by one or more drugs. On top of that some diabetic stroke patients do not respond to standard dose thrombolytic therapy and are extremely difficult to treat [29]. Considering the previous information and the refractory phenomenon seen in some diabetic patients, questions are raised if using a standard naive-hypertensive rodent will be adequate to anticipate drug efficacy in a heterogeneous population of stroke patients [29]. Some preclinical studies used spontaneous hypertensive or diabetic rats but no significant improvement in behavioral or histologic outcome was seen when the results of these studies were pooled [35-38]. This observation does support the use of more complex experimental models before translation to clinic.
3 studies conducted stem cell transplantation in aged rodents. We know that advanced age is associated with a decrease in brain plasticity and higher vulnerability to ischemic damage. Aged rats thus respond differently to experimentally induced stoke. They had larger infarct volume and higher overall mortality than younger animals and they also had a poorer functional recovery and significant decrease in angio- and neogenesis. Despite the differences in age, transplantation of stem cells resulted in significant improvement in both young and old animals, so the aging-related micro-environment does not impede an effective response to stem cell transplantation [39-41].
The guidelines set by STEPS, which also provide a basis for exploring stroke animal models for translation of cell-based therapy, emphasize the use of appropriate species, including larger animals. They also stated that clinically relevant stroke animal models, preferably in an NHP, are desirable for testing the potential of anti-stroke therapeutic strategies. There is still a requirement to improve the many existing animal models, so they can correctly reflect our ability to test possible clinical treatments [31].
When choosing an adequate cell type, two important considerations need to be considered. First, stem cells can be divided into autologous or allogeneic stem cells. Allogeneic transplantation implies the chance for host immunity and graft-versus-host disease, while the risk for immune reactions is avoided when using autologous stem cells. The need for immunosuppressant when using allogeneic stem cells might be an important hurdle in the clinical practice of stem cell transplantation. Secondly, all cell types have the need for in vitro, or if possible in vivo, expansion to meet he adequate injection dose and the time and requirements needed to expand each cell type differ between cell types.
One of the most frequently used cell types in preclinical studies are mesenchymal stem cells (MSC). These cells are multipotent and are most commonly isolated from bone marrow and adipose tissue, but can be derived from amniotic fluid, the placenta and the umbilical cord as well. Both adipose- and bone marrow-derived MSCs have shown to be effective in preclinical trials. MSCs have the advantage of not carrying a risk for tumorigenesis, like pluripotent cells, and being immunoprivileged, with low major histocompatibility complex (MHC) expression. MSCs even have an immunosuppressive nature, which obviates the need for immunosuppressant in allotransplantation. Adipose tissue contains more than a thousand-fold more MSC compared with bone marrow, so preparing enough cells is much easier for adipose-derived stem cells. Harvesting of bone marrow-derived MSCs also involves a highly invasive procedure, while adipose-derived stem cells use a less invasive method. Additionally, the age, sex and drug usage of cell donors have a great influence on the quality of bone-marrow derived MSCs. Adipose tissue-derived MSCs are thus considered to be a more convenient option than bone marrow-derived MSCs [42-44].
Since MSCs require complicated culturing conditions for in vitro expansion, some studies used peripheral blood hematopoietic stem cells (PBSC). These are autologous stem cell without the need for in vitro expansion. After several days of granulocyte colony stimulating factor (G-CSF) injections, PBSC can be easily harvested from the bloodstream, so no invasive extraction procedure is needed [45,46].
Another type of frequently used stem cell is human umbilical cord blood cells (HUBCB). HUBCB have the advantage of easy availability as it is discarded post birth and lack of ethical conflicts. Since HUBCBs are allogeneic, they require HLA-matching, which limits their clinical application. However, one study declared that there is a tolerance to HLA-mismatch with non-to low severity from HLA mismatch [38].
In total, more than 20 different cell types have been used in our included preclinical studies, all with their advantages and disadvantages. The difference between autologous and allogeneic cell transplants seemed of major importance for clinical translation, but since evidence suggested that several allogeneic stem cells can be administered without immunosuppression. Still, the included clinical trials all used autologous stem cells. At present, there is no study comparing all these different cell types in a direct manner, so it is difficult to advise one specific cell type for clinical application. Embryonic cells have ethical concerns, limited availability and the potential of tumor formation. Induced pluripotent stem cells also have the possibility of late oncogenesis and long-term safety is still a serious problem. So, although it is too early to withdraw conclusions, these cell types are unlikely to be proposed in the future [47,48].
To determine the correct cell dose, preclinical studies that investigate cell dose-response curves are necessary. Researchers observed dose-dependent immune-suppressive effect in vitro, so cell dose might play a crucial role in the effective translation of preclinical evidence [35].
When we evaluate studies, using different cell doses, most often a higher dose was more effective than a lower dose [49-51]. A single high dose of 3 × 106 cells is considered better than multiple low dose injections at different times [52]. One study declared that 2 different administrations were better than one single administration [35]. But here, the benefit is possible explained by the absolute higher dose caused by the double injection, rather than the double injection itself. The dose-response curve probably follows a u-shape rather than a linear progression, with higher number of cells leading to a ceiling effect. After all, too large doses of stem cell are observed to be destructive and therefore have diminished effects. One study described a severe ipsilateral eye inflammation followed by acute mortality of study animals after administration of 107 cells. Mesenchymal stem cells have the tendency to aggregate in multicellular globules, thus at higher concentrations could result in vascular embolization. This study indicated 5 × 106 cells as the maximum number of cells that could be safely transplanted [53]. This result was confirmed be the study of Yang, et al. were the injection of 5 × 107 cells caused high mortality rates due to embolisms, so 5 × 106 cells were seen as ideal cell dose therapy [54]. Another study showed that animals died when they received greater than 30 × 106 cells, stating 10-20 × 106 cells might be the optimal range [36].
Lower doses of cells did not manage to get enough cells entering the brain for effective treatment, so too low cell doses are also thought to be ineffective [13,50,51]. However, there are several studies with low cell doses (≤ 105) that showed a significant improved function [42,44,55-58].
One study did not find any dose-response relationship suggesting that inadequate dose is not a likely explanation for failure of translation to clinic [18].
Different cell doses have been used in the included RCTs. Based on mean body mass, Bang, et al. computed that 1 × 108 cells/patient is the human dose equivalent to the dose used in rodent stroke models. They considered 1 × 105 - 3 × 106 cells/rat as the effective dose used in rodents [59]. This dose was injected by Lee, et al. as well [60]. The other two clinical trials used different doses so the optimal dose of stem cells in human stroke model still needs to be determined and high-quality dose-response studies are urgently needed. Another important point of investigation is whether the number of cell passages might downgrade the efficacy of stem cells. When extrapolating rodents' doses to humans, large numbers of cells are needed. This means that at least 5 or 6 or more passages are needed to achieve a sufficient dose [51,61]. If the efficacy of stem cells diminishes by more cell passages, this could partly explain the lack of efficacy of stem cell therapy in humans.
Besides the possible importance of the number of cells, the proposal has been made that cell size and velocity of injection are of equal value [62]. Slower injection might diminish sludging and thereby allow higher cell doses [44].
The most appropriate route for administration still needs to be determined. Preclinical studies use mainly three different injection routes: Intracerebral (IC), intra-arterial (IA) and intravenous [IV]. In the included RCTs stereotactic and intravenous administration were used.
IC transplantation results in the highest number of cell deposits into the ischemic brain and is shown to be an effective administration route. However, this is also the most invasive route and studies have demonstrated that the stereotactic inoculation of cells can cause damage. IC injection can thus be potentially complicated by hemorrhage or injury, probably by needle insertion, as well as fluid loading, neuronal cell deaths, reactive gliosis and micro-calcification [36,63-65].
Both intravascular routes are less invasive and can avoid unnecessary brain damage. IA transplantation has been correlated with a decrease in infarct volumes, functional recovery and a high number of surviving stem cells. IA injection however, holds the risk of cerebral embolisms and reduction of blood flow with the occurrence of micro-occlusions when high doses of stem cells are infused [62,65]. IV injection is the less-invasive, simplest and practical route of administration, but has the disadvantage that there is a dispersal of cells throughout the body. Fewer cells reach the brain parenchyma because they get entrapped in the lungs and other peripheral organs [54,62,65].
IC administration is unambiguously the least applicable route in clinical setting, since it involves an invasive procedure with high risks of additional damage and possible neurological worsening of the patient's state. If IC injection is chosen, safety trials have opted for delivery of cells many months or years after stroke at the earliest. Patients need to be stable to undergo general anesthesia for this invasive procedure and no spontaneous recovery may be expected [28]. However, one of the included RCT used stereotactic injection of stem cells and this appeared to be safe [46]. In contrast to IC admission, the recommendations regarding intravascular administration routes are less straightforward and differ between several preclinical studies.
Some studies, comparing different administration routes, stated that IA and IC delivery resulted in a higher number of surviving stem cells and a higher improvement in histologic and behavioral outcome [13,44,66]. Other studies did not find a significant influence of route of administration on the efficacy of cell transplants. IA and IV infusion both resulted in improved functional status and/or histologic outcome with no added benefit of IA administration [12,14,50,51].
One study suggested that the number of cells delivered to the brain may be not critical to influence functional outcomes or that only a minimum threshold of cells delivered to the brain is needed and that higher cell presence does not lead to a greater effect on recovery in rodent stroke models. This would explain how IV delivery, with lesser cells achieving the brain, can be as effective as IA delivery [50]. This hypothesis is enhanced by the fact that paracrine effects of stem cell-associated growth factors are considered to be the main effective pathway [36].
Based on preclinical evidence, the three administration routes can be effective, so the route of delivery might not be the major hurdle in translating preclinical evidence to clinical practice. IV injection is sometimes seen as the least effective route, but clearly has some great safety and practical advantages. We presume that IV injection should be the route of predilection to consider in clinical practice, like 3 out of 4 RCTs [18,59,60] already did.
Several studies have compared different administration times. For example, the study of Komatsu, et al. delivered stem cell in 3 time periods from 7 days after middle cerebral artery occlusion (MCAo) until 28 days after MCAo. The stem cells injected at 7 days post-MCAo did significantly better than the cells transplanted at 28 days. Administration at 4 weeks post-MCAo might thus be too late to be effective [49]. The study of Nam, et al. on the other hand, compared 1 h, 1 day or 3 days post-MCAo injection. The 1 h post-MCAo transplanted group showed maximum neurological recovery, in both functional and structural way [61]. Other studies stated that 24 h is the most optimal administration time, because this might go along with the time of opening of the blood-brain barrier [44,65,67].
In general, preclinical evidence mostly supports early administration [52,56,68,69]. This early transplantation, however, has practical complications in clinic setting, especially when autologous stem cells are used which need time for expansion. Delayed inoculation of cells is a better representation of the planned clinical scenario, where disability following stroke will need to be stabilized before treatment [49]. It is therefore beneficial that several preclinical studies show that late administration of stem cells still can be effective. 14 of the included studies delivered stem cells at 1 week or later after MCAo. 13 of these studies, including some in which stem cells were only administered 4 weeks after MCAo, reported an improvement in behavioral and/or histologic outcome [45,49,55,63,68,70-78]. The other study did not report significant improvement in histologic outcome and even worsening of behavior was noted [62].
When defining an optimal delivery time for clinical practice, several factors, besides the preclinical evidence, must be considered. First, pilot studies have shown that many patients undergo marked improvement or worsening in the first week after stroke. When deterioration is severe, they might even need hemicraniectomy, which would mask the possible effect of cell transplantation. Secondly, cytokines released from the intervention might destabilize the patients in the first week and cause serious adverse effects, which would be unethical to perform [18,60]. Lastly, after a sudden stroke, there is time needed to prepare autologous stem cells, which can only be delivered several weeks later [64]. So even though preclinical evidence suggests early stem cell transplantation, this is impossible in clinical practice. The present RCTs offered cell therapy to stroke patients after weeks and months of stroke onset. This late administration might be a possible explanation for lack of benefit, but the current available knowledge there is no possible manner to transplant cells at earlier time points. The exact optimal time is still unknown, so additional studies concerning the time of transplantation seem necessary [28,59]. However, since histologic and functional improvement have been described for cell transplantation time points from 1 h to 1 month upon MCAo, the time window for effective stem cell transplantation might eventually be less relevant [14,35].
Despite immense advancements, a myriad of factors is hampering the acceptance and the survival of stem cell. Within hours, a very high number of donor cells die after transplantation [79]. This high mortality is incriminated to several pathological processes that encompasses local immunological and inflammatory responses, loss of trophic factors as well as local primary factors responsible for the initial ischemic insult (e.g. reduced perfusion and nutrients, hypoxia and others) [80,81].
Improvement of stem cell transplantation is related to the number of cells retained and remained active at the site of the cell graft. Increasing the number of cells engraftment may overcome the massive cell death and washout, but number of complications remain such cell aggregation, vascular embolism, inflammation generated by cell death of the graft [36,44,53,54].
Hence, it is imperative to reinforce the donor cell to withstands the rigors of the microenvironment of the infarcted site by optimizing cell engraftment and this without increasing complications [82]. Authors had shown in stroke [83] and in myocardial infarction [80], that priming cells prior transplantation with cytokines to a state of "readiness" by stimulating their survival pathways will fortify cell engraftment leading to enhance survival and to overcome numbers of problems occurring during the post engraftment period.
Stem cell therapy seems promising in animal models, but before extrapolation to humans large and well-designed preclinical trials are needed. We consider preclinical study quality as one of the main factors for failed translation to clinic. Designing high-quality studies should focus first on the optimal cell type, dose, route of administration and timing of delivery. These issues were noticed by researchers in the field as well and gave rise to the several guidelines, but currently their effect on study quality is still lacking. Some researchers state that several issues concerning the translating of stem cell therapy to human, might be better directly addressed by careful studies in human stroke models, rather than supplementary preclinical studies [13]. This possible approach is supported by the excellent safety record of our included clinical trials.
If stem cell therapy is going to be used in clinic, research needs to be done to determine which stroke patients need to be considered. Because, it is possible that the efficacy of stem cell transplantation will be affected by the location, severity and chronicity of the stroke and the competence of blood supply [59]. Another strategy for the future is the investigation of modified stem cells. Several preclinical studies already showed that modified stem cell might have better functional outcomes than unmodified cells. Gene transduction into stem cells is a possible manner to enhance their current therapeutic potential [14,84-86].
Our study has some limitation since only 4 RCTs using human stroke models could be included in the systematic review. Although this is much more than the Cochrane meta-analysis in 2010, the sample sizes of the included studies are small, so our results are not conclusive. Our research should be repeated when more high-quality studies are available.
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter of materials discusses in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.