We present a patient who developed post kidney transplant renovascular hypertension due to Fibromuscular dysplasia, which was unseen on donor's renal artery angiography. The temporal relationship to the clinically manifested hypertension and the patient's profound weight loss makes us postulate her intra-abdominal fat redistribution led to nephroptosis of the transplanted kidney, causing stretching of the renal artery, exaggerating the Fibromuscular dysplasia and hemodynamically significant stenosis. This phenomenon of traction-stretch of an artery leading to hemodynamical significant stenosis been described in the past, but to our best knowledge is the first time described as possible cause of transplant renal artery stenosis. Prompt diagnosis and early referral for percutaneous intervention allowed our patient to recovery from hypertension.
Hypertension, Renal, Angioplasty, Fibromuscular dysplasia, Renal artery obstruction, Kidney transplantation
Hypertension is a common problem in transplanted kidney recipients; it is associated with allograft failure and cardiovascular complications. In addition to the general risk factors and recipient's underlying condition(s) that prompted kidney transplantation, recipients encounter additional risks related to the donor, immunotherapy and allograft dysfunction.
In the general population, atherosclerosis is the leading cause of renovascular hypertension, followed by Fibromuscular Dysplasia (FMD) [1,2]. FMD is an idiopathic, noninflammatory, nonatherosclerotic vascular disease, which mainly affects young women, with a reported incidence in potential kidney donors of 2-6% [3,4]. With the advances in non-invasive imaging, renal artery stenosis is identified prior to organ donation. We present a patient who developed post kidney transplant renovascular hypertension due to Fibromuscular Dysplasia, which was unseen on donor's renal artery angiography.
A 48-year-old female with accelerated end-stage renal disease due to Good pasture syndrome underwent living related kidney transplant from her younger sister 7-years-ago. Prior to nephrectomy, her sister had a normal computed tomographic angiography of the renal arteries. Her postoperative period was unremarkable and optimal immunosuppression was achieved with 3 mg of twice-daily Tacrolimus and 1 g of twice-daily Mycophenolate Mofetil (MMF). She had stable creatinine levels of 0.6 mg/dl with glomerular filtration rate above 90 ml/min and remained normotensive. Ten-months-ago, after returning from a trip to Asia, she began to experience watery stools, and anorexia. She underwent extensive work-up and tested positive Strongyloides stercoralis in the absence of eosinophilia or stool parasite. She completed a standard treatment course, with non-significant relief of her symptoms and gradual weight loss of 20 pounds (17% body weight).
Subsequently, a colonic biopsy was consistent with drug-induced colitis; her dose of MMF was decreased to 250 mg twice a day and 1 mg of Sirolimus was started. Within 2 weeks, her symptoms resolved but during the next follow-ups her blood pressure started to rise. She was started on low doses of Losartan and a 24-hours blood pressure monitor revealed an average pressure of 150/98 mmHg. A Doppler ultrasound of the transplanted kidney revealed a 13.6 cm kidney in the pelvic fossa (no prior ultrasound was available); peak systolic velocities were obtained and showed: Iliac artery 148 cm/s, transplanted Renal artery anastomosis 113 cm/s, mid-Renal artery segment 551 cm/s and hilar segments 517 cm/s.
The patient was referred for renal angiography, which revealed a focal web-like stenosis in the mid portion of the transplanted renal artery (Figure 1). A Fractional Flow Reserve (FFR) wire was advanced through the stenosis and a 45 mmHg gradient was noted across the lesion. Subsequently, a 5 × 20 mm RX Viatrac 14 Plus balloon (Abbott Vascular, Inc., CA, US) was crossed and inflated to its nominal pressure (Figure 2). Post-angioplasty (Figure 3), the systolic gradient across the stenosis decreased to 25 mmHg. More aggressive dilatations were not performed because of the proximity of the stenosis to the bifurcation of the renal artery. A decrease in the blood pressure was noted in the recovery room and remained controlled without antihypertensive therapy in the subsequent visits.
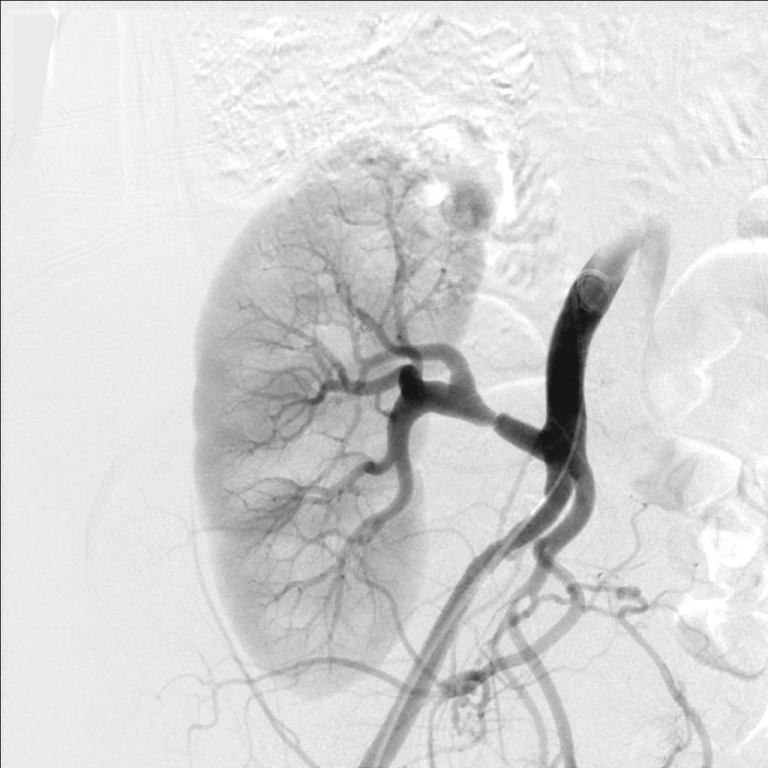 Figure 1: Unselective iliac artery angiography, using a pigtail catheter, showing the transplanted renal artery stenosis in its mid portion. View Figure 1
Figure 1: Unselective iliac artery angiography, using a pigtail catheter, showing the transplanted renal artery stenosis in its mid portion. View Figure 1
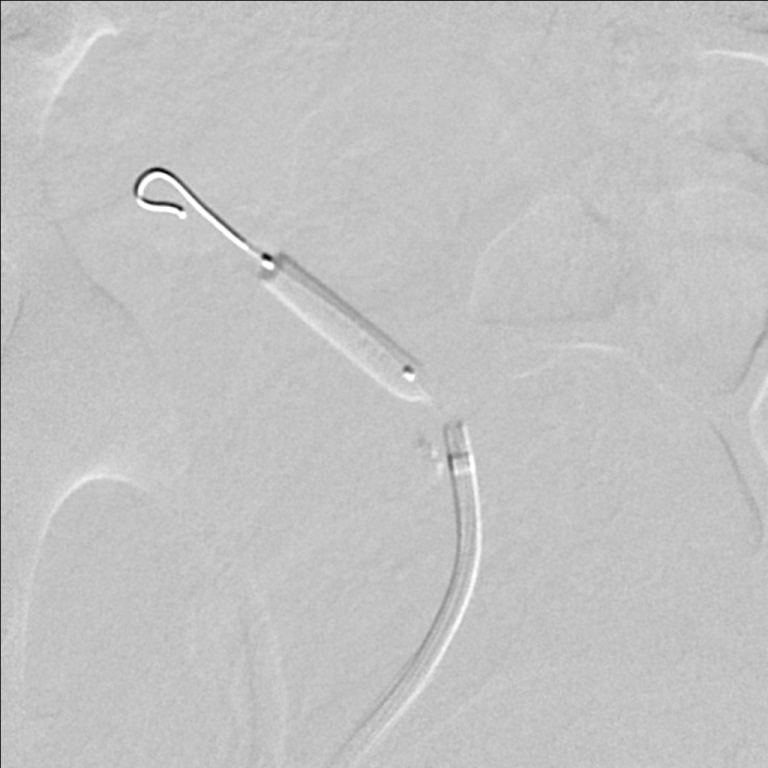 Figure 2: A 5 × 20 mm RX Viatrac 14 Plus balloon (Abbott Vascular, Inc., CA, US) is used to cross the lesion and inflated to its nominal pressure. View Figure 2
Figure 2: A 5 × 20 mm RX Viatrac 14 Plus balloon (Abbott Vascular, Inc., CA, US) is used to cross the lesion and inflated to its nominal pressure. View Figure 2
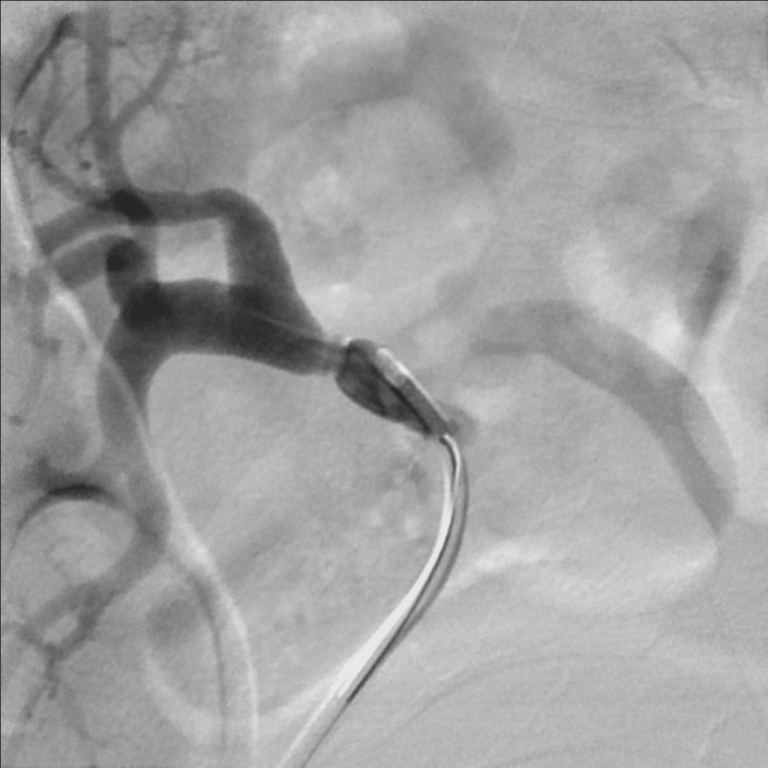 Figure 3: Selective angiography of the transplanted renal artery after transluminal angioplasty. View Figure 3
Figure 3: Selective angiography of the transplanted renal artery after transluminal angioplasty. View Figure 3
Transplant Renal Artery Stenosis (TRAS) is a challenging complication reported in 1% to 23% of recipients leading to renovascular hypertension [5,6] and often caused by accelerated vascular disease. Surgical technique (vessel trauma, malposition of the kidney, prolonged cold ischemic time), atherosclerosis and immunologic factors are possible triggers for it. Moreover, FMD is a non-atherosclerotic disease of the medium vessels [3,7] that affects the renal artery and results in focal stenosis and aneurysms involving not only the proximal but also its mid-distal portion [1,5]. It manifests most commonly as hypertension but impaired renal function, renal infarction or atrophy are also seen.
In this case, FMD was most likely insignificant and clinically silent in the donor. The temporal relationship to the clinically manifested hypertension and the patient's profound weight loss makes us postulate her intra-abdominal fat redistribution led to reaccommodation of the transplanted kidney in the pelvic fossa. The movement of the kidney in response to a change in the posture has been termed nephroptosis or Floating kidney. Although this entity is infrequent in native kidneys, is even rarer in transplanted kidneys. Nevertheless being female, obese, and having lax abdominal walls have all been reported to be predisposing factors.
It is then possible that nephroptosis, in the case of our patient, caused stretching of the renal artery provoking hemodynamically significant stenosis due to a silent FMD. The phenomenon of traction-stretch of an artery with FMD leading to hemodynamical significant stenosis has been described in the past [8], but to our best knowledge, is the first time described as possible cause of TRAS.
For years FMD has been classified in three main subtypes according to histopathological examination [1,7]. In the current practice, advances in noninvasive imaging studies have shown a significant sensitivity to detect it and tissue sampling is rarely needed. Duplex ultrasonography in presence of FMD, typically reveals a step-up in peak systolic velocity in the mid to distal portion of the main renal artery, as seen in our case, or a delayed systolic upstroke (tardus et parvus waveform) in arterial branches distal to the stenosis [3]. Nevertheless, the gold standard is catheter-based angiography with the current angiographic classification supported by the American Heart Association dividing it into two subtypes: Multifocal and focal [3].
Since the introduction of peripheral artery angioplasty, few patients with FMD have undergone primary surgical revascularization [2], as FMD responds well to Percutaneous Transluminal Angioplasty (PTA) [3,7]. Most treatment decisions in patients with FMD are based on data derived from single case reports or small retrospective case series.
Patients who are treated with balloon angioplasty alone or angioplasty with stenting are treated in the same way as patients with atherosclerotic disease who have undergone intervention. According to the Scientific Statement From the American Heart Association [3], revascularization for FMD, by Percutaneous Transluminal Angioplasty (PTA) or surgery should be considered in: 1. Resistant hypertension (failure to reach goal blood pressures in patients on an appropriate 3-drug regimen including a diuretic). 2. Hypertension of short duration with the goal of a cure of hypertension. 3. Renal artery dissection; rarely is intervention needed, but if so, stenting is generally the procedure of choice. 4. Renal artery aneurysm(s); surgical resection, endovascular coiling, or placement of a covered stent is usually used. 5. Branch renal artery disease and hypertension; some lesions can be treated with PTA, but if this is not possible, surgical revascularization may be required, often with bench repair. 6. Preservation of renal function in the patient with severe stenosis, especially in the pediatric population with perimedial fibroplasia or intimal fibroplasia.
It is important to mention that the negative trials like ASTRAL [9] and CORAL [10] on stent implantation for atherosclerotic renal artery disease do not apply to patients with FMD given the differing pathophysiology and natural history of these 2 vascular disorders [3]. In contrast to atherosclerotic disease, primary stenting of the renal artery is not recommended in FMD. Furthermore, young patients and shorter duration of hypertension are good prognostic indicators after revascularization in renal FMD [11], therefore percutaneous angioplasty may be considered first-line therapy in young patients, early in the course of the disease with the goal of cure. The development of restenosis or worsening hypertension should lead to consideration of angiography and repeat PTA [3].
Living donation requires careful and accurate evaluation of renal anatomy, morphology, and vascular supply in part to determine which kidney is the safest to remove and which has the best function and should remain with the donor [12]. Occasionally, failure to recognize mild-to-moderate and mostly mid to distal renal arterial stenosis, particularly Fibromuscular Dysplasia (FMD), in donor kidneys can cause hypertension, renal insufficiency and ischemic renal failure, both in the recipient if transplanted with lesion and in the donor if the remaining artery has stenosis postdonation [4,13]. Prompt diagnosis and early referral for PTA allowed our patient to recovery from hypertension.
A major limitation to support that nephroptosis was the sole cause of hemodynamically significant stenosis is the absence of objective evaluation of the transplanted kidney before and after weight loss, as this is not part of the routine evaluation of a transplanted patient. More so, did not evaluate positional changes of the transplanted kidney as our clinical suspicion and treatment let to clinical improvement. We consider that a more objective evaluation should be considered in where nephroptosis is considered.
None.
Dr. de Marchena, Director of the Scientific Advisory Board; Tendyne Medical Inc, Baltimore, MD, USA. The rest of the authors do not have anything to disclose.