We present an autopsy case of a 66-year-old man who died during walking. Besides a history of hypertension for 5 years, mild chest oppression appeared to occur shortly before death. A full autopsy and the police examination concluded that the cause of death was sudden cardiac death. The coronary artery showed a myocardial bridge in the left descending coronary artery and two bridges in the right coronary artery. Concentric hypertrophy of the left ventricle and moderate atherosclerotic narrowing of the coronary artery other than muscular bridges were found. Histological examination of the left ventricle showed minimal, but obvious, acute necrotic foci in addition to multiple small myocardial scars and advanced interstitial fibrosis. Multiple myocardial bridging may be a potential factor for sudden cardiac death in association with other factors, especially with left ventricular hypertrophy. Careful multilateral examination of the heart may be essential for evaluating the clinical significance of a muscular bridge when pathologists encounter sudden cardiac death with this relatively frequent anomaly.
Autopsy, Coronary artery, Myocardial bridge, Sudden death
A Myocardial Bridge (MB) is a congenital anatomical structure consisting of myocardial tissue that covers a part of the coronary artery in the epicardial adipose tissue. Although the frequency of an MB varies in the literature, it has a relatively higher incidence than the other anomalies [1-3]. MB is most frequently observed in the Left Anterior Descending Artery (LAD), but has also been found in other branches, such as the left circumflex artery and Right Coronary Artery (RCA). Multiple MBs have also been found in a limited number of cases [3,4]. MB has been found in many non-cardiac deaths. Therefore, this anomaly may tend to be evaluated as benign. However, some clinical studies have shown a decrease in systolic blood flow in MBs of symptomatic patients [5], and autopsy cases of sudden cardiac death with this anomaly have been rarely reported [6-9]. We report here a case of sudden cardiac death with multiple MBs and other possible pathological appearances.
A 66-year-old man was found dead on the sidewalk near his house. A police examination showed that he left his friend's home 3 hours before discovery and was on his way home. He had a history of hypertension and was routinely prescribed β-blockers for approximately 5 years. He did not have an experience of athlete, and his family doctor commented that he recently and rarely complained of mild precordial discomfort. A recent resting electrocardiogram showed nonspecific ST-T wave changes. An additional examination was not performed before death partly because of the victim's inactive attitude.
The man's height was 178 cm and body weight was 68 kg. Autopsy showed only some mild abrasions of the face and forearm. A toxicological examination was negative. His heart weighed 520 g and was examined according to previously reported methods [10]. Careful dissection of pericardial adipose tissue showed MBs in the LAD and RCA. The proximal RCA and right ventricular branch were covered by muscular tissue. The length of MB was 2.5 cm in the LAD, 0.7 cm in the proximal RCA, and 0.6 cm in the right ventricular branch. Symmetric concentric hypertrophy of the Left Ventricle (LV) was found, and the thickness was 2.0 cm in the LV free wall and 2.1 cm in the ventricular septum (Figure 1). The thickness of MBs was 25 mm in the LAD and 12 mm in two MBs of the RCA. Coronary dominance of the heart was co-dominant.
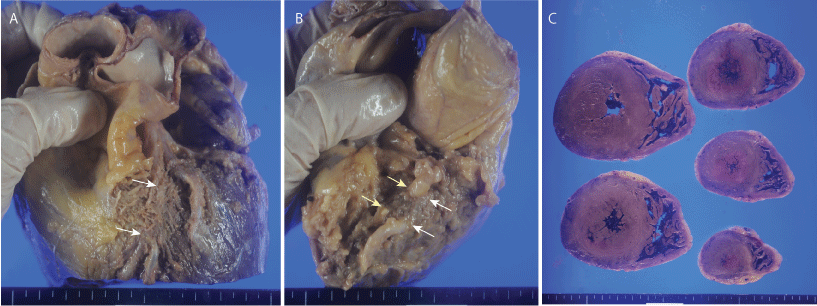 Figure 1: Gross appearance of the heart. A) Muscular bridge of the left anterior descending artery is indicated by arrows; B) Muscular bridges in the right coronary artery. Yellow arrows indicate a muscular bridge of the proximal right coronary artery, and white arrows indicate a muscular bridge of the right ventricular branch; C) Horizontal section of the ventricle shows concentric hypertrophy of the left ventricle.
View Figure 1
Figure 1: Gross appearance of the heart. A) Muscular bridge of the left anterior descending artery is indicated by arrows; B) Muscular bridges in the right coronary artery. Yellow arrows indicate a muscular bridge of the proximal right coronary artery, and white arrows indicate a muscular bridge of the right ventricular branch; C) Horizontal section of the ventricle shows concentric hypertrophy of the left ventricle.
View Figure 1
Microscopic examination of MBs showed mild intimal thickening and interstitial fibrosis of circumferential adipose and cardiac muscle. A mild to moderate degree of atherosclerosis was found in the coronary artery tree other than the area of MBs. Approximately 25 to 50% stenosis was found in the proximal area of the left circumflex artery and the first diagonal branch derived from the LAD. Multiple minute fibrous scars were found in the entire left ventricle by histological examination. A few small, acute, necrotic foci with appearance of some inflammatory cells were also found in the LV (Figure 2). The cardiac conduction system did not show any major pathological changes. No other pathological lesion that could cause sudden cardiac death was evident in the brain, lungs, and other organs.
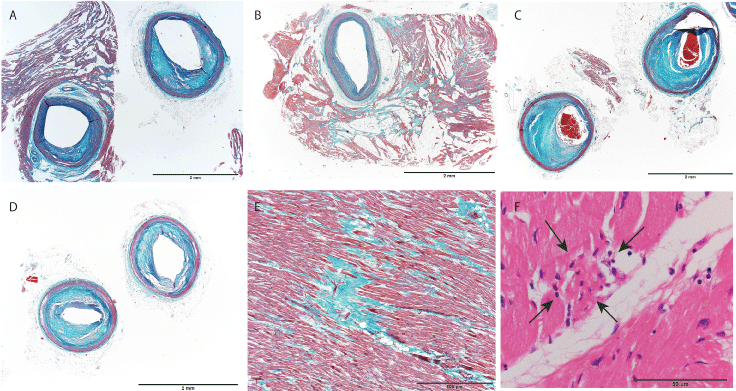 Figure 2: Histological appearance of the heart. A) Left anterior descending artery. The left panel shows the arterial wall just proximal region from the entrance of the muscular bridge. The right panel shows the artery within the muscular bridge (Elastica-Masson staining); B) Muscular bridge of the right coronary artery. Advanced fibrosis around the MB was found (Elastica-Masson staining); C,D) Moderate atherosclerotic narrowing of the left circumflex artery; C) and the first diagonal branch derived from the left anterior descending artery (Elastica-Masson stain); E) Micro scar and interstitial fibrosis of the left ventricle (Elastica-Masson stain); F) Small acute necrosis with inflammation in the left ventricle is indicated by arrows (hematoxylin and eosin staining). Scale bar = 2 mm (A-D), 500 μm (E), 50 μm (F).
View Figure 2
Figure 2: Histological appearance of the heart. A) Left anterior descending artery. The left panel shows the arterial wall just proximal region from the entrance of the muscular bridge. The right panel shows the artery within the muscular bridge (Elastica-Masson staining); B) Muscular bridge of the right coronary artery. Advanced fibrosis around the MB was found (Elastica-Masson staining); C,D) Moderate atherosclerotic narrowing of the left circumflex artery; C) and the first diagonal branch derived from the left anterior descending artery (Elastica-Masson stain); E) Micro scar and interstitial fibrosis of the left ventricle (Elastica-Masson stain); F) Small acute necrosis with inflammation in the left ventricle is indicated by arrows (hematoxylin and eosin staining). Scale bar = 2 mm (A-D), 500 μm (E), 50 μm (F).
View Figure 2
Impairment of the coronary circulation within MBs by compression from the surrounding myocardium during systole is a significant factor for occurrence of clinical complications. These complications include myocardial ischemia [11], acute coronary syndrome [12,13], coronary spasm [14,15], myocardial stunning [16], arrhythmia with syncope [17,18], and sudden death [6-9]. Clinical significance of MB for sudden cardiac death has not been fully established yet because the border between a pathological or non-pathological MB has been unequivocal yet. However we should note the recent report that surgical treatment can significantly improve the severity or frequency of angina related symptoms suggests the pathological MBs are present at a constant rate [19].
The incidence of MB in previous autopsy studies varied from 5% to 86%, and 13.5% be recently found in a study by Teofilovski-Parapid, et al. [3]. They also showed multiple MBs in only one of 96 examined hearts. Loukas showed a low incidence of multiple MBs, especially in cases with multiple MBs in different coronary trees, as in the present case (four of 69 hearts with MB) [4]. Findings from the present case showed that multiple MBs may have been an additional risk for occurrence of significant myocardial ischemia. The clinical significance of coronary dominance in cases of MB is controversial. Teofilovski-Parapid, et al. commented that coronary dominance is ethnically specific and may not be significantly correlated with the presence of MB and possible occurrence of associated ischemia [3].
Some studies have suggested that a MB of the LAD with a long and/or deep course may cause pathological hemodynamic impairment in the MB because of vulnerability in receiving mechanical force from circumferential cardiac muscle. Alegria [20] and Bourassa [21] showed that the clinically significant length and thickness of a MB are 10 to 30 mm and 2 to 4 mm, respectively. In particular, a deep variant in which the MB in the LAD deviates toward the right ventricle and dives into the intraventricular septum may be a relatively high risk of severe compression to the MB. This could be caused by additional systolic compression from the muscle bundle arising from the right ventricular apical trabeculae. The superficial variant is more common in MBs of the LAD (approximately 75% of MB cases) [22]. The MB of the LAD in the present case could be significant anomaly based on the length and depth of the MB.
The presence of many asymptomatic patients with MB suggests that a symptomatic decrease in blood flow within the MB may occur in selected patients. Therefore, additional pathophysiological changes in the coronary artery or heart may be required to decrease blood flow within the MB and associated myocardial ischemia. Corban, et al. showed that four associated potential factors could cause pathological ischemia in cases of MB. One possible factor is an increase in left ventricular diastolic dysfunction that could cause an additional decrease in blood flow within the MB. Aging, hypertension, and atherosclerosis of the coronary artery may be causes of diastolic dysfunction. A second possible factor is an increase in compression and reduction in the coronary microvascular reserve by left ventricular hypertrophy. A third possible factor is coronary vasospasm and microvascular dysfunction of endothelial dysfunction. A fourth possible factor is progression of atherosclerosis proximal to the MB. In the present case, progression of left ventricular hypertrophy might have caused additional diastolic dysfunction of the LV and diminished blood flow by compression to the MB [23]. The pathological significance of fibrosis around the MB could not be determined. However, we speculate that fibrosis is a tissue reaction against continuous compression to the MB. An additional case study may be required.
The degree of atherosclerosis in the present case was not advanced. However, some autopsy and clinical studies have shown that the proximal segment of a MB is prone to developing atherosclerosis, despite being free from atherosclerosis within the MB segment and the distal segment [24,25]. Fluid dynamics play an important role in plaque formation at the entrance of an MB. Previous studies have shown that low and oscillatory wall shear stress at the entrance of an MB compared with within a MB could cause various pathological and physiological changes, leading to atherosclerosis. Enhanced myocardial compression to an MB and/or subsequent deformation and additional unusual motion of the coronary tree other than an MB are also associated with atherosclerosis of the area [22,26].
Pathologists have difficulty in diagnosing sudden cardiac death due to MB as a conclusive autopsy diagnosis when the pathological appearance of old and/or fresh ischemia are not evident. Careful examination targeting signs of ischemia in cardiac muscle may be essential for determining the clinical significance of MB for sudden death. Beside many small areas of myocardial scarring and extensive interstitial fibrosis, small, but obvious, fresh ischemic necrosis was found in the present case by careful examination. We cannot conclude whether multiple MBs of the present case were a unique pathological condition for his sudden death. However, at least multiple MBs may have been a major predisposing factor because advanced myocardial ischemia was evident, despite mild to moderate atherosclerotic narrowing of the coronary tree. Associated acquired myocardial hypertrophy and mild exertion may decrease the amount of blood flow within multiple MBs, and might increase the chance of an arrhythmogenic event. Marron, et al. showed that MB was not significantly associated with occurrence of sudden cardiac death in cases with hypertrophic cardiomyopathy [27]. However, they did not exclude the possibility that MB increases the risk in some cases. Findings from the present case showed that careful multilateral evaluation for the heart may be useful for evaluating the clinical significance of MB in each autopsy case when sudden unexpected death is encountered with this relatively frequent anomaly.
In summary, we cannot conclude that multiple MBs can cause sudden unexpected death as a unique factor. However, findings from the present case show that multiple MBs may be a major potential factor for causing sudden unexpected death in certain people. Besides anatomical and pathological evaluation of MB, detailed pathological examination targeting both additional findings could diminishing the blood flow within MBs, such as myocardial hypertrophy and/or atherosclerotic narrowing of the coronary tree may be essential. Findings suggestive of advanced ischemia in the heart may be essential for determining the degree of the pathological significance of MB in each case.
This work was supported in part by a KAKENHI grant from JSPS, Japan, to Y.H. (JP15k08867).
None.