Atrial Fibrillation (AF) is the most common cardiac arrhythmia and a cause of increased morbidity and mortality. Left Atrial (LA) tissue characteristics play an important role in the pathogenesis of AF. Catheter ablation of AF has emerged as a popular treatment option, however predictors of treatment success are not clearly defined. For a better understanding of the LA structural organization we noninvasively characterized Left Atrial Wall Thickness (LAWT) and Pulmonary Vein Cross-Sectional Area (PVCSA) using Multidetector Computed Tomography (MDCT) in AF patients and healthy controls.
We included 47 patients with paroxysmal/persistent AF scheduled for catheter ablation and 47 age, Body Mass Index (BMI) and gender matched healthy controls without any history of cardiac disease. All participants underwent 320-MDCT scan for assessment of LAWT and PVCSA.
LAWT ranged from 0.4 to 3.0 mm and was significantly larger in all 12 preselected locations in AF patients (33-64%) compared to healthy controls (mean thickness 1.7 ± 0.3 vs. 1.1 ± 0.3 mm, p < 0.0001). In both groups significant regional anatomical differences were found; mainly the left lateral ridge differed as a specific thicker region in both groups.
PVCSA ranged from 32 to 616 mm2 per vessel and was significantly enlarged in AF patients (16-41%) compared to healthy controls (total summed pulmonary veins cross-sectional area 1052 ± 238 vs. 809 ± 155 mm2 p = 0.004). In both AF patients and controls the right superior pulmonary vein was largest. .
Atrial fibrillation is associated with universally increased left atrial wall thickness and pulmonary vein size when compared to healthy controls.
Atrial fibrillation, Left atrium, Multi-detector computed tomography, Catheter ablation.
Atrial Fibrillation (AF) is the most common cardiac arrhythmia and a cause of increased morbidity and mortality [1]. AF treatment consists in preventing thrombo-embolic complications and in treatment of symptoms using anti-arrhythmic therapy or frequency control [1]. Catheter ablation in the Left Atrium (LA) has become a therapeutic option to maintain sinus rhythm in patients with AF, although the substrates for the development of AF and therefore the target for ablation remain to be fully described [1].
The pulmonary veins are central to the initiation of the fibrillatory process, as it defines an area of increased electrical activity [1]. The centrepiece in ablation is consequently the creation of transmural LA wall lesions that ensure permanent electrical isolation of the pulmonary veins [2].
Alterations in LA geometry and wall tissue composition may play a critical role in arrhythmia induction and perpetuation [3]. The LA cavity and the Pulmonary Veins (PV) are complex anatomical structures with important interpatient variability in size, form and branching patterns [4,5]. The interaction between LA anatomy and ablation catheters including contact force, catheter stability, energy delivery intensity and duration of ablation, has been shown to affect the clinical efficacy of ablation [2].
MDCT is a well established modality for pre-ablation evaluation of gross anatomy of the LA with focus on PV location and numbers [4]. However, recent developments in MDCT have made it possible to visualize the LA in greater details, including wall characteristics and the PV size noninvasively with high spatial and reasonable temporal resolution by creating high-quality three-dimensional images [6]. This widely accessible imaging modality may be used to provide a more detailed anatomical evaluation and road map before ablation, in terms of both LA and PV anatomy [3,7-12]. Recent studies indicate that LA and PV characteristics as determined by cardiac MDCT are possible important predictors of AF recurrence after ablation therapy [11,13-16], and that the thicker the parts of the LA are, the less successful ablation therapy becomes [13,17]. In addition, it has been found that patients with persistent AF have enlarged LA cavity, decreased left ventricular function and lower success rates than other patients referred for ablation [7,9,18,19].
The aim of this study was to investigate associations between AF and LA and PV anatomy using MDCT in patients with AF and healthy controls.
This study was cross-sectional. Study population was recruited among consecutive patients scheduled for ablation for paroxysmal/persistent AF at Rigshospitalet, University of Copenhagen. The AF patients were included based on the following criteria: age > 18 years and referred to ablation for paroxysmal/persistent AF - both initial and repeat ablation. Patients were excluded if they were known to have allergy to iodine contrast, had an estimated GFR below 50 ml/min, were unable to understand the study information or had significant valvular heart disease.
The controls were recruited from the Copenhagen General Population Study (CGPS), which is an on-going prospective general population study with registration of health-related conditions [20]. Participants were randomly selected, and age-, BMI- and gender-matched in sinus rhythm without a history of cardiac disease in a ratio of 1:1 with AF patients. The Danish National Committee of Biomedical Research Ethics approved the study protocols and all patients gave written informed consent.
MDCT was performed prior to ablation in the AF patients group and used as image integration to guide the ablation. Cardiac imaging for both groups was performed using a 320 MDCT scanner (Aquilion One, Toshiba Medical systems). Automated bolus triggering in the descending aorta with a threshold of 170 Hounsfield Units was used for initiation of image acquisition, and 50-80 ml intravenous contrast agent (Visipaque 320, GE Healthcare) was infused (flow rate of 5.5 ml/s) followed by a saline chaser. The scanner settings were: gantry rotation time 0.275 s and detector collimation 0.5 mm × 320. Tube voltage was 100 kV and current ranging between 40 and 490 mA based on the participants’ BMI. A cardio selective beta-blocker (metoprolol 25-150 mg) was administered orally 1 h before scanning in controls with a heart rate of more than 60 bpm, unless systolic blood pressure was less than 110 mmHg or other contraindications to beta-blocker treatment did exist. For controls a fixed so-called target protocol using one rotation acquisition with a fixed prospective window of 350 ms centred at the 75% phase of the RR-cycle was used. For the AF patients retrospective gating, dose modulation and iterative reconstruction algorithms were used. Adaptive Iterative Dose Reduction 3D (AIDR 3D) is the newest iterative reconstruction algorithm developed by Toshiba which - based on the scanning conditions and electronic noise statistics - preserves the maximal attenuation density signal while noise and artifacts are reduced. The estimated radiation dose was 5 mSv. Raw data were reconstructed in 5% intervals throughout the cardiac cycle, with a slice thickness and increment of 0.5/0.5 mm. All image data were transferred to an external workstation (Vitrea 6.2, Vital Images Inc, MN, USA) for image analysis. The MDCT reader, the LAWT interobserver and the PVCSA interobserver had 2-3 years of MDCT image analysis experience including training from experienced supervisors and relevant courses.
Assessment of left atrial wall thickness, pulmonary vein size and left atrial volume were performed using the mid-diastolic frame with least cardiac motion as assessed automatically from raw-data.
It was measured from axial views in 12 preselected locations (Figure 1) based on the differences in Hounsfield Units (HU) for various compartments (contrasted blood 200-600 HU, left atrial wall tissue 50 HU and pericardial fat - 100) and with anatomical fix points (the pulmonary veins and the left atrial appendage) as guidance in location determination (Figure 2). We included 3 locations at the roof (right, middle, left), 3 locations at the floor (right, middle, left), 4 locations at posterior wall (right, middle, middle-superior, left), 1 at the Left Lateral Ridge (LLR), and 1 at the mitral isthmus as previously described [11]. The LAWT was assessed as the mean of 3 manually performed measurements in each preselected location, or excluded if not possible due to anatomical anomalies or motional artefacts - 23 measurements (2.0%) in the AF group and 52 measurements (4.7%) in controls out of a total of 1128 measurements. Global LAWT was calculated as the mean thickness in each patient based on a summed mean of all preselected locations. In addition regional differences in LAWT and relative thicknesses (location thickness/mean thickness) were evaluated and compared between the two groups.
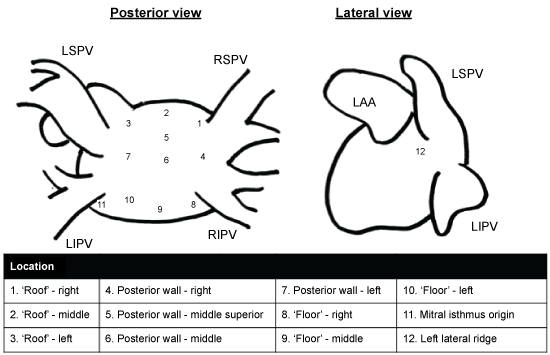 Figure 1: Preselected locations for left atrial wall thickness assessment, modified from Beinart, et al. [11]. LIPV: Left Inferior Pulmonary Vein; LSPV: Left Superior Pulmonary Vein; RIPV: Right Inferior Pulmonary Vein; RSPV: Right Superior Pulmonary Vein.
View Figure 1
Figure 1: Preselected locations for left atrial wall thickness assessment, modified from Beinart, et al. [11]. LIPV: Left Inferior Pulmonary Vein; LSPV: Left Superior Pulmonary Vein; RIPV: Right Inferior Pulmonary Vein; RSPV: Right Superior Pulmonary Vein.
View Figure 1
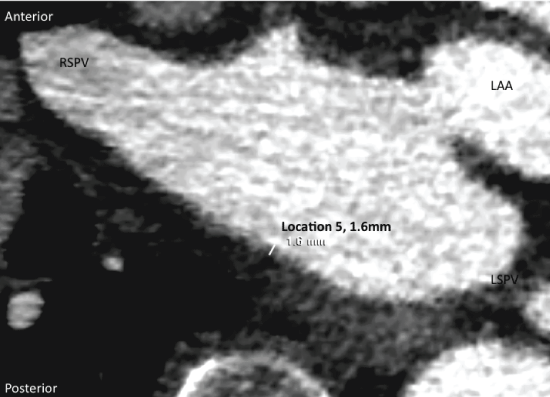 Figure 2: Method demonstration of left atrial wall thickness assessment, in this case location 5. Axial view. RSPV: Right Superior Pulmonary Vein; LAA: Left Atrial Appendage.
View Figure 2
Figure 2: Method demonstration of left atrial wall thickness assessment, in this case location 5. Axial view. RSPV: Right Superior Pulmonary Vein; LAA: Left Atrial Appendage.
View Figure 2
It was defined as the cross-sectional area of each pulmonary vein 5 mm perpendicular to the ostium of the pulmonary vein (Figure 3). The PV ostium was defined as the intersection between the LA wall and PV wall, and the extent of the ostium was determined using multiple coronal planes. The pulmonary veins were assumed to be ellipsoidal, and the cross-sectional area was calculated using the formula for the area of an ellipse: area = p×a×b, where a and b was manually measured in a coronal oblique sectional view including the particular pulmonary vein. The pulmonary veins identified in this study were; Left Common Pulmonary Vein (LCPV), Left Superior Pulmonary Vein (LSPV), Right Superior Pulmonary Vein (RSPV), Left Inferior Pulmonary Vein (LIPV), Right Inferior Pulmonary Vein (RIPV) and Right Middle Pulmonary Vein (RMPV). The PVCSA was also assessed as the total summed Pulmonary Veins Cross-Sectional Area (PVSUM-CSA).
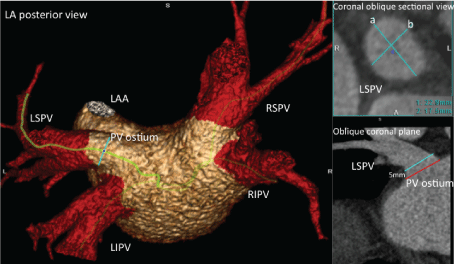 Figure 3: Method demonstration of pulmonary vein cross-sectional area assessment. a and b refers to the formula for the area of an ellipse: area = p×a×b. LIPV: Left Inferior Pulmonary Vein; LSPV: Left Superior Pulmonary Vein; RIPV: Right Inferior Pulmonary Vein; RSPV: Right Superior Pulmonary Vein; LAA: Left Atrial Appendage.
View Figure 3
Figure 3: Method demonstration of pulmonary vein cross-sectional area assessment. a and b refers to the formula for the area of an ellipse: area = p×a×b. LIPV: Left Inferior Pulmonary Vein; LSPV: Left Superior Pulmonary Vein; RIPV: Right Inferior Pulmonary Vein; RSPV: Right Superior Pulmonary Vein; LAA: Left Atrial Appendage.
View Figure 3
It was measured from axial view by manually tracing the endocardial wall on 15-20 tomographic slices as previously described (interobserver variation 1.5% (SD: 6.6%)) [8]. The pulmonary veins were identified and excluded from the PV ostium, whereas the LA appendage was included in the LA cavity. The border of the LA was defined as a straight line between the attachments of the mitral valve. The software interpolates the endocardial tracings and calculates the absolute volume of the LA cavity. LAV was also indexed by Body Surface Area (BSA), LAVI (LAV/BSA). LAV indices are previously described [21-23].
Interobserver variability evaluation was performed for both LAWT and PVCSA, and intraobserver variability for LAWT. For assessment of interobserver variability, the two observers were each blinded to the clinical status of each individual patient and to the measurements of the other observer. To evaluate intraobserver variability for LAWT, one observer measured 20 AF patients and 20 controls randomly selected, blinded by its own measurements, 3 times during the MDCT analysis with 2-3 weeks in-between.
Categorical data are expressed as frequencies and percentages, and continuous and normally distributed data by their mean ± standard deviation. Differences between two groups were tested with the unpaired t-test. Differences between locations were assessed with one-way ANOVA, hereunder the Tukey’s Studentized Range comparison. To evaluate the associations between LAWT and gender, hypertension, hypercholesterolemia, duration of AF and type of ablation multivariate linear regression analyses were performed. Degree of agreement between observers was assessed with the Bland-Altman difference against mean plot, and inter-/intra-observer variability as the mean percentage error. Correlation of PVSUM-CSA was further assessed with Pearson’s test of correlation. A p-value of less than 0.05 was considered significant for all analyses. Statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary, North Carolina, USA).
A total of 137 AF patients were screened (September 2013 to May 2014) and 47 patients included. Patients were excluded if they had significant valvular heart disease (n = 20), had an estimated GFR below 50 ml/min (n = 20), were unable to understand the study information (n = 5) or were known to have allergy to iodine contrast (n = 2), and there to 42 declined participation or did not respond, and 1 had a non-diagnostic CT scan. Demographic information of patients and controls enrolled in the study is given in Table 1. There was no significant difference in BSA, hypertension, tobacco and creatinine between patients with AF and controls. Of the 47 patients with AF 39 (83%) had paroxysmal AF, and the mean duration of AF disease was 5 years. In 79% it was a first-time AF and in 21% a repeat scheduled catheter ablation procedure. AF during CT scan was present in 14 (30%) patients.
Both observers found a statistically significant difference in LAWT in AF patients compared to controls, with 95% limits of agreement from -1.2 mm to 0.5 mm (n = 94). Yet comparison between the two observers revealed significant systematic difference in mean thickness (0.5 mm in the AF patients, p-value < 0.0001 and 0.1 mm in the controls, p-value 0.04). The intraobserver variability showed no significant difference in measurements with 95% limits of agreement from -0.7 mm to 0.7 mm (n = 20).
For assessment of interobserver variability for PVCSA, two observers measured 10 AF patients and 10 controls randomly selected, each blinded to the measurements of the other. The agreement between the two observers for PVSUM-CSA was a correlation of 0.72 with a variability of 0.51.There was no significant difference between the PVSUM-CSA as measured by the two observes with 95% limits of agreement from -299 mm2 to 233 mm2.
Patients with AF had significantly higher LAV compared to controls (141 ± 28 and 79 ± 22 ml, p-value < 0.0001) and also when indexed by BSA (69 ± 13 and 39 ± 8 ml/m2, p-value < 0.0001). No significant association was found between LAV and LAWT.
LAWT in the 12 predefined anatomic locations for patients with AF and controls are given in Figure 4. In all locations the LAWT was thicker in the AF patients compared to controls (p < 0.0001). The mean LAWT was 1.7 ± 0.3 mm for patients with AF, and 1.1 ± 0.3 mm for controls (p < 0.0001), and there was no difference in mean LAWT between AF patients with sinus rhythm and with AF during MDCT (1.7 ± 0.2 mm vs. 1.6 ± 0.3 mm, p = 0.46).
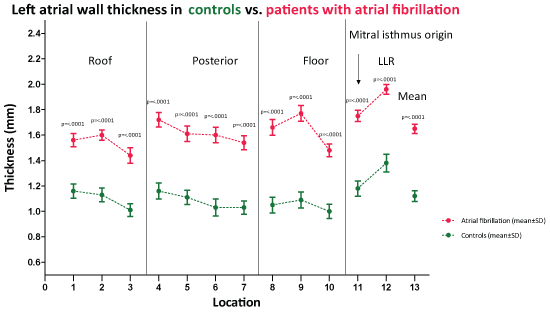 Figure 4: Graphical representation of left atrial wall thickness measured by MDCT in 12 preselected locations including mean thickness in controls and patients with atrial fibrillation. Values are mean. LLR: Left Lateral Ridge. View Figure 4
Figure 4: Graphical representation of left atrial wall thickness measured by MDCT in 12 preselected locations including mean thickness in controls and patients with atrial fibrillation. Values are mean. LLR: Left Lateral Ridge. View Figure 4
No significant association was found between mean LAWT and gender, hypertension, or hypercholesterolemia in either of the groups. Neither any correlation between LAWT and age was found. Furthermore, in AF patients, no association was found between LAWT and duration (years of AF), type of AF (paroxysmal or persistent) or type of ablation (first-time or repeat) (Table 1, Table 2 and Supplementary File).
Supplemental Table 1: Differences on LAV, LAWT and PVCSA in type of AF. View Supplemental Table 1
Supplemental Table 2: Differences on LAV, LAWT and PVCSA in type of ablation. View Supplemental Table 2
Table 1: Demographics. View Table 1
In both groups location 12 (left lateral ridge) was significant thicker compared to the other locations. There was no significant difference in degree of relative thickness between AF patients and controls.
PVCSA in patients with AF and controls are shown in Figure 5. All pulmonary veins and PVSUM-CSA (1052 ± 238 vs. 809 ± 155 mm2 p = 0.004) were significantly enlarged in AF patients compared to controls. There was no difference in mean PVSUM-CSA between AF patients with sinus rhythm and with AF during MDCT (p = 0.41). The number of pulmonary veins identified in AF patients and in controls was respectively: 44 and 46 LSPV, 44 and 46 LIPV, 47 and 47 RSPV, 47 and 47 RIPV, 3 and 1 LCPV and 4 and 1 RMPV. No comparison of PVCSA for LCPV and RMPV was possible due to the low number of observations. No significant association was found between mean PVCSA and age, gender, hypertension, or hypercholesterolemia in either of the groups. No association was found between PVCSA and duration (years of AF), type of AF (paroxysmal or persistent) or type of ablation (first-time or repeat) (Table 1, Table 2 and Supplementary File).
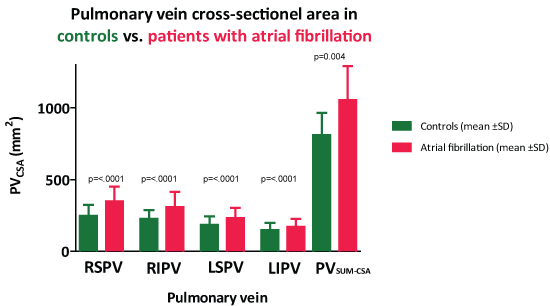 Figure 5: Pulmonary vein cross-sectional area in controls and patients with atrial fibrillation. Values are mean ± SD. LIPV: Left Inferior Pulmonary Vein; LSPV: Left Superior Pulmonary Vein; RIPV: Right Inferior Pulmonary Vein; RSPV: Right Superior Pulmonary Vein.
View Figure 5
Figure 5: Pulmonary vein cross-sectional area in controls and patients with atrial fibrillation. Values are mean ± SD. LIPV: Left Inferior Pulmonary Vein; LSPV: Left Superior Pulmonary Vein; RIPV: Right Inferior Pulmonary Vein; RSPV: Right Superior Pulmonary Vein.
View Figure 5
The relative differences between patients with AF and controls with regards to mean LAV, LAWT and PVSUM-CSA are given in Figure 6.
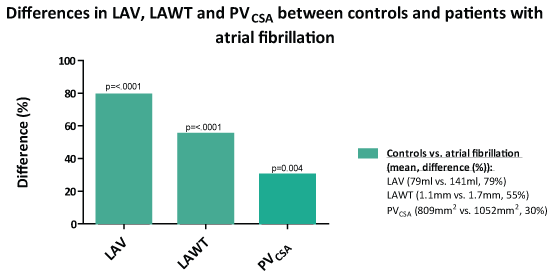 Figure 6: Percentage difference in mean left atrial volume, left atrial wall thickness and pulmonary vein cross-sectional area between patients with atrial fibrillation and controls. LAV: Left Atrial Volume; LAWT: Left Atrial Wall Thickness; PVCSA: Pulmonary Vein Cross-Sectional Area.
View Figure 6
Figure 6: Percentage difference in mean left atrial volume, left atrial wall thickness and pulmonary vein cross-sectional area between patients with atrial fibrillation and controls. LAV: Left Atrial Volume; LAWT: Left Atrial Wall Thickness; PVCSA: Pulmonary Vein Cross-Sectional Area.
View Figure 6
Catheter ablation in AF is well recognized as an antiarrhythmic therapy, however the difficulty and variability of the left atrium remains technically challenging, and inherent to this method is the risk for serious complications including cardiac tamponade, stroke and atrio-esophageal fistulas. Consequently there has been an increasing interest in improving ablation techniques through a better understanding of LA anatomy, and with the current advancements in MDCT it is feasible to depict the LA in great detail. We investigated in this study the anatomy of the LA with MDCT and demonstrated that paroxysmal or persistent AF is associated with a substantially increased LAWT, PVCSA and LAV, when compared to healthy age and gender matched individuals.
LAWT was obtained manually in 12 locations generally targeted in ablation and a regional heterogeneity comparable to what was found in other studies was noted. We found that AF is associated with a uniformly increased wall thickness in 12 anatomical locations of the left atrial chamber when compared to healthy controls. Previous studies have found differences in LAWT between patients with AF (both persistent and paroxysmal) and controls [3,6,14], but none of them describe a population with healthy matching controls as this. Nakamura, et al. [14] describe in their study, that patients with paroxysmal AF have significantly increased LAWT (2.4 ± 0.2 mm) compared to subjects with either persistent AF (2.1 ± 0.2 mm) or sinus rhythm (1.9 ± 0.2 mm). Dewland, et al. [3] describe oppositely thinner LA walls in patients with AF compared to controls. Beinart, et al. [11] investigated LAWT in a group of patients with persistent AF with no comparison to controls and described a large regional variety of LAWT (0.5-3.5 mm) with an average thickness of 1.9 ± 0.48 mm, which is comparable with our AF cohort. As Beinart, et al. [11] and as well histological studies [24,25] we also found significant regional differences in both groups; predominantly location 12 stood out as a specific thick region compared to the rest of the LA in both patients with AF and controls. Location 12 covers anatomically the left lateral ridge; a well-known structural complex region often re-ablated [24]. In general LAWT for controls in this study was thinner compared to previous studies [3,14,26]. Yet the control cohorts are characterized by much diversity and size variability.
Currently there is no consensus on the exact method in LAWT determination using MDCT. Previous studies have sought to develop simple and automated algorithms [3,6], yet larger cohorts and procedural ablation outcomes are needed.
This study is to our knowledge the first to investigate PVCSA as a parameter in LA anatomy description in AF patients referred to ablation compared to healthy matched controls. Prior studies have investigated PV anatomy with focus on branching pattern, composition, size and ridges [15,16,27]. For comparison, McLellan, et al. [16] describe in their study the dimensions of the PV ostia in a group of patients with AF with similar size distribution as in our study. They find PVCSA for RSPV, RIPV, LSPV and LIPV comparable to our AF cohort. In both studies right-sided PVs are larger than left-sided, and in both superior veins are larger than inferior veins. Different from our study McLellan, et al. are not comparing PV dimensions with controls.
We introduce novel information about to what extent the structure of the LA and PV anatomy is altered in AF patients compared to healthy subjects in sinus rhythm. Prior and larger population studies have described LA and PV anatomy in patients with AF [13-16,28,29].
In ablation the creation of LA transmural lesions and sufficient contact-force are fundamental success criteria [2,30,31], and pre-procedural information about LAWT, regional differences, PVCSA, PV variants and LAV allow the ablation operator to customize and individualize treatment and ablation strategy to ensure durable PV-isolation. In each case, procedure focus could be directed towards the thickest parts of the LA, which may require most intensive ablation and also the thinnest areas, where perforation risk and risk of collateral damage may be higher. In addition, future studies will determine whether local variation in LA and PV anatomy will be able to predict specific areas of later electrical reconnection and the need for re-ablation. Also pre-procedural electrocardiographic parameters might be interesting to examine, hereunder the relationship between LA anatomy and ECG p-wave length in successful ablation, as described by Truong, et al. [32].
The mechanisms behind alterations in LAWT, PVCSA and LAV shown by MDCT remain to be determined, however it is most likely that these changes may point to the occurrence of atrial remodelling, hypertrophy, inflammation, fibrosis etc. Further studies should investgate, whether an increased atrial wall thickness or larger pulmonary vein area could predict outcome after ablation.
This study has a number of limitations. Firstly the number of patients is relatively low. Secondly, the AF patients consisted of both paroxysmal and persistent AF and initial and repeat ablation, and a complete description of prior therapy approaches is missing. Thirdly only 47 out of 137 AF patients were included and the AF history in the control group was unknown. The interobserver variability for LAWT was considerably high reflecting the complexity in technique, and underlines the importance of great caution and guidance when using this method. Nonetheless both observers find a significant alteration in LAWT in AF patients compared controls, the intraobserver variability is low, and the mean thickness is in our opinion a solid and reliable value based on a substantial amount of measurements.
Our study indicates that left atrial wall thickness, pulmonary vein cross-sectional area and left atrial volume measured with multidetector computed tomography are uniformly increased in patients with atrial fibrillation compared to healthy age and gender matched controls without any history of cardiac disease.
The present study is the first to investigate pulmonary vein size and left atrial wall thickness in AF patients compared to healthy controls without any history of cardiac disease
• We found that patients with AF have significantly thicker left atrium walls compared to healthy controls in 12 predefined anatomical locations.
• We found a significant enlargement in pulmonary vein cross-sectional area in patients with AF compared to healthy controls.
• Whereas left atrial wall thickness was increased by 55%, pulmonary veins were only increased by 30%.
This study is approved by the Danish National Committee of Biomedical Research Ethics and complies with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. All patients were consent to the additional radiation due to the study protocol.
Klaus F Kofoed reports being on the Speakers Bureau of Toshiba Medical Systems and doing Advisory board work for VITAL Images Inc. Peter K Jacobsen reports receiving speakers fee from St Jude Medical. Anna F Thomsen, J Tobias Kühl, Andreas Fuchs, Patricia M Udholm, Jakob B Norsk, Xu Chen, Steen Pehrson, Børge G Nordestgaard and Lars Køber have no conflict of interest to declare.
This work was supported by ‘Lawyer Bent Thorberg Foundation’ ‘A.P. Møller and wife Chastine Mc-Kinney Møller Foundation’ ‘The John and Birthe Meyer Foundation’ and further financial support was provided from ‘The Heart Centre Research Foundation, Rigshospitalet’.
The authors thank Christina Møller, Kirsten Thrysøe, Stella Lauenborg Hansen, Jane Ellingsgaard Poulsen, Michael Below and Elsebeth Olsen for their invaluable technical assistance.