Perioperative opioid sparing techniques are paramount given the current opioid epidemic. A 54-year-old woman presented with medical history of acute cholecystitis for laparoscopic cholecystectomy. Our goal was adequate pain control while limiting opioid administration and opioid-related adverse effects. Pain was managed with preoperative acetaminophen, tramadol, and gabapentin, and intraoperatively with lidocaine, ketamine, and esmolol. Post-operative Visual Analog Score (VAS) was 0/10 immediately following surgery. Although her pain score peaked to 10/10 30 minutes post-operatively and she received 0.4 milligrams intravenous hydromorphone, her VAS then declined to 0 and remained so throughout her hospitalization without additional analgesics.
VAS: Visual Analog Score; MAC: Minimum Alveolar Concentration; PACU: Post-Anesthesia Care Unit; NMDA: N-methyl-D-aspartate; n: Number of subjects; TAP: Transversus Abdominis Plane; LA: Local Anesthetic; LAI: Local Anesthetic Infiltration
Perioperative opioid sparing techniques are requisite given the current opioid epidemic. Post-operative pain control remains a significant issue for laparoscopic cholecystectomy. Inadequate pain control is frequently the main complaint postoperatively, the reason for prolonged hospital stay, prolonged recovery, and potentially the development of chronic pain [1]. Postoperative nausea and vomiting, sedation, urinary retention, sleep disturbances, and respiratory depression are among many of opioid-related adverse effects, and may contribute to increased in-hospital morbidity and costs [2]. As such, opioid-sparing modalities have been employed to improve pain control and recovery, and minimize opioid related adverse effects.
In a systematic review of randomized controlled trials, findings showed perioperative intravenous lidocaine infusion resulted in significant reductions in postoperative pain intensity and opioid consumption compared to controls for open and laparoscopic abdominal surgery [3]. A randomized controlled trial examining perioperative intravenous lidocaine infusion during laparoscopic cholecystectomy found that those who received the lidocaine infusion had significant reduction in time for bowel recovery, and less cytokine release, compared to controls [4].
Studies demonstrating the effect of lidocaine in conjunction with other opioid sparing modalities such as esmolol and ketamine are needed. We present a case in which we achieved an opioid free anesthetic for laparoscopic cholecystectomy utilizing a combination of lidocaine infusion, esmolol, ketamine, and perioperative multimodal medications. Our goal was adequate pain control while limiting opioid-related adverse effects.
This manuscript adheres to the applicable EQUATOR guideline. HIPAA authorization has been obtained from this patient.
A 54-year-old woman with no past medical history presented with right-upper-quadrant and epi-gastric pain, nausea and vomiting. Evaluation showed 1.2 cm dilation of the common bile duct on ultrasound, and increasing liver profile studies. After a thorough work up by the general surgery and gastrointestinal services, the patient underwent an elective laparoscopic cholecystectomy for gallstone pancreatitis and acute cholecystitis.
Prior to the procedure, the patient reported a Visual Analog Score (VAS) of 0/10 pain. She had not taken any pain medication within 2 days of surgery. The risks and benefits of general anesthesia were discussed with the patient, and she agreed to proceed with surgery under general anesthesia. Multimodal PO medications including 1 gram Tylenol, 50 milligrams tramadol, and 100 milligrams gabapentin were given preoperatively.
Prior to induction of anesthesia, the patient received 2 milligrams of midazolam. Anesthesia was induced with 60 milligrams lidocaine, 30 milligrams ketamine, 100 milligrams propofol, 60 milligrams succinylcholine, and 40 milligrams esmolol. After intubation the patient was maintained with 1.0 Minimum Alveolar Concentration (MAC) Sevoflurane. A lidocaine infusion was started immediately after induction and run at 2 milligrams/minute until extubation (total 80 minutes, ~158 milligrams). Prior to incision, the surgeon injected each of the 4 port sites with 0.25% bupivacaine and no drains were placed for postoperative drainage. The patient received10 milligrams ketamine and 20 milligrams Esmolol each hour and 30 milligrams of ketorolac at the end of the case. She remained hemodynamically stable throughout the procedure and emergence. She was extubated and taken to the Post-Anesthesia Care Unit (PACU) for monitoring.
The patient reported a VAS of 0/10 on admission to the PACU. At 30 minutes postop, her VAS peaked at 10/10. The patient received a total of 0.4 milligrams hydromorphone in the first 45 minutes after her surgery. VAS were 6/10, 4/10 and 0/10 by 45 minutes, 1 hour and 2 hours respectively. VAS thereafter remained 0/10 until discharge the following day. The patient did not require any additional opioid or multimodal pain medications during her hospitalization.
In an attempt to limit opioid use, multiple medications with different effects on pain pathways were employed. Opioids primarily act through the inhibitory descending pathway to decrease neuronal excitability and nociceptive neurotransmitter release in the central and peripheral nervous systems.
In contrast, ketamine exerts its effects by antagonizing the N-methyl-D-aspartate (NMDA) receptor at the level of the spinal cord to modulate nociceptive processing and hyperexcitability [1]. NMDA receptors have been shown to play a role in inflammatory pain, secondary hyperalgesia, neuropathic pain, and chronic pain as a result of sensitization via spinal neural plasticity occurring secondary to persistent pain [5]. In the setting of surgery, noxious stimuli activate C-fiber nociceptors, which triggers glutamate release and NMDA receptor activation [6]. Ketamine blocks this pathway thereby decreasing neuroexcitation, with the added benefit of reducing the development of chronic pain by inhibiting neuronal sensitization. At low doses such as that used for our patient (0.5 milligram/kilogram), ketamine has been shown to reduce postoperative pain with minimal to no adverse effects [6].
Lidocaine has a multifactorial approach to pain modulation. Due to a low blood concentration achieved with a dose of 1-2 milligrams/hour, it is unlikely related to sodium channel blockade (as it could not block all sodium channels at such a low dose) but rather provides analgesia through mediation of inflammatory signaling pathways. The proposed mechanism involves local anesthetic blockade of polymorphonuclear granulocytes (which do not express sodium channels) from producing reactive oxygen species and cytokines that leads to inflammation and subsequent pain [7].
Esmolol is a short acting β-1 receptor antagonist used perioperatively to manage tachycardia and hypertension. It is posited that esmolol may play a role in central analgesia, via either stimulation of G-protein coupled receptors controlling the release of neurotransmitters, or by blocking hippocampal activation of N-methyl-D-aspartate subtype glutamate receptors, via adrenergic pathways, which may attenuate the perception of pain [8,9]. Esmolol has shown opioid sparing benefit in doses ranging from 1.0 microgram/kilogram up to 2 milligram/kilogram bolus, followed by infusion ranging from 5-500 micrograms/kilogram/minute. In our patient, esmolol was given as a bolus of 40 mg (equivalent to roughly 0.7 milligram/kilogram) at induction, then 20 milligram each hour thereafter throughout the surgery, which totaled to an additional 40 milligram.
To evaluate the efficacy of our pain management regiment, we compared our patient's post-operative pain scores to those published by other studies investigating multimodal pain regimens in patients specifically undergoing laparoscopic cholecystectomies (Figure 1). We also compared opioid consumption over 24 hours post-op in our patient and the mean of those receiving the other pain regimens (Figure 2).
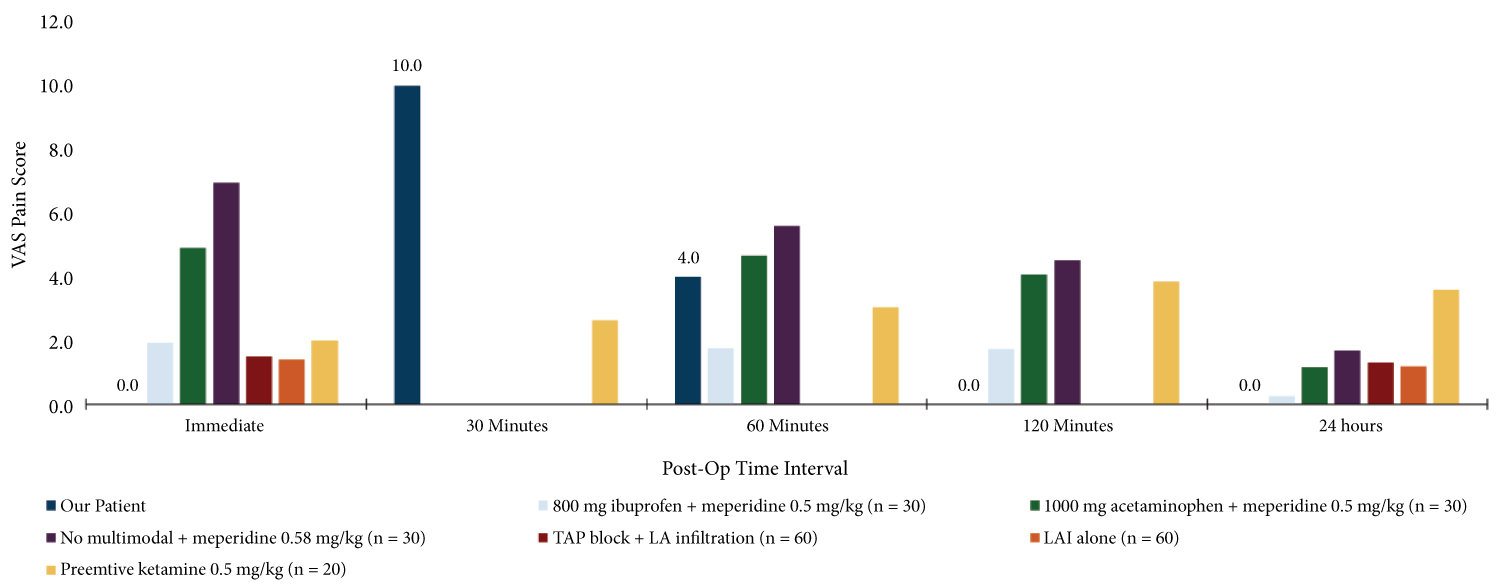 Figure 1: Postoperative VAS in patients receiving different analgesic regimens periopertively. Bar graph reflects mean VAS for each pain regiment per study.
Figure 1: Postoperative VAS in patients receiving different analgesic regimens periopertively. Bar graph reflects mean VAS for each pain regiment per study.
n = number of subjects.
View Figure 1
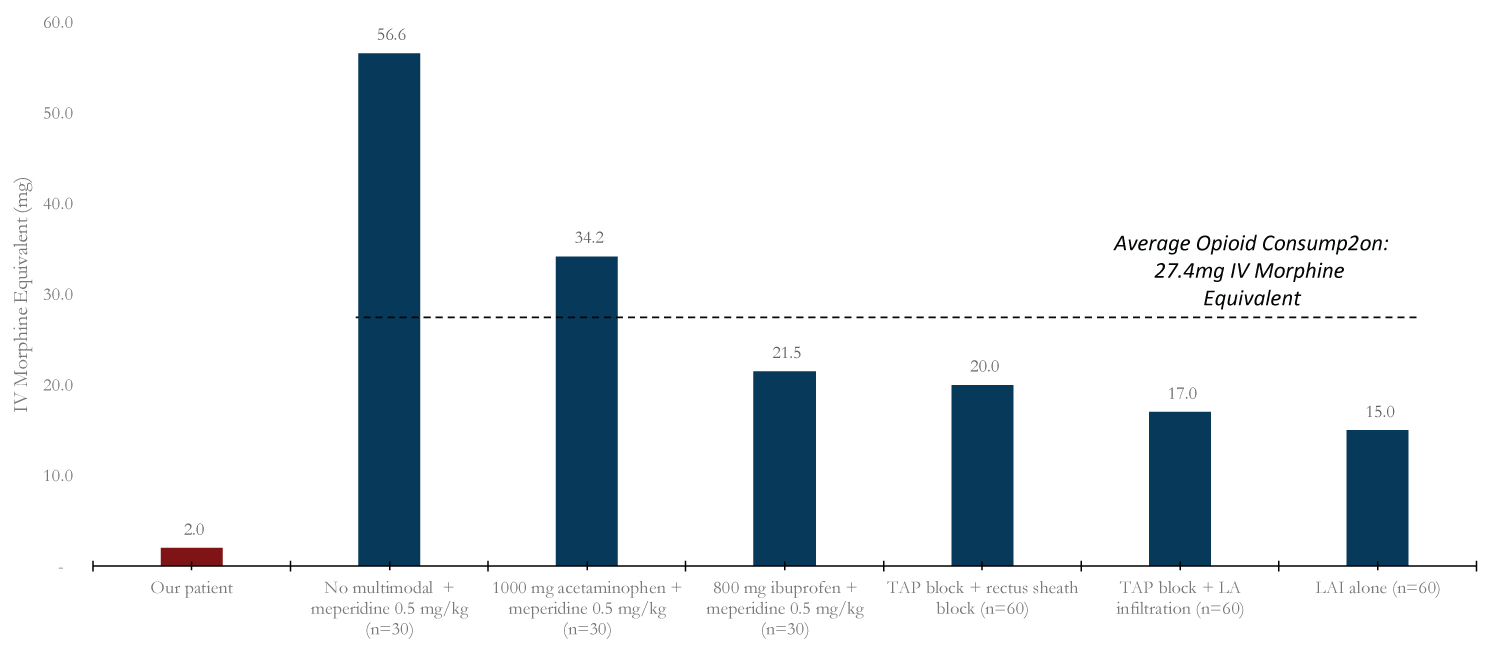 Figure 2: 24-hour opioid consumption. Bar graph reflects mean opioid consumption in 24 hours for each pain regimen per study. Opioids used per study converted to intravenous morphine equivalent.
Figure 2: 24-hour opioid consumption. Bar graph reflects mean opioid consumption in 24 hours for each pain regimen per study. Opioids used per study converted to intravenous morphine equivalent.
n = number of subjects.
View Figure 2
As listed in Figure 1, the pain management regimens investigated in other studies included various combinations of ibuprofen, acetaminophen, and meperidine; another compared local anesthetic infiltration (LAI) and transversus abdominis plane (TAP) block; and a third looked at how the addition of ketamine affected VAS [6,10,11]. Taking a look at our patient's post-operative pain scores (depicted in dark blue) on this first graph, we can see that immediately postop, our patient reported no pain. Her pain score did peak to 10/10 at 30 minutes, but then declined thereafter, remaining at 0 at 2 hours and through discharge the following day.
Table 1 illustrates post-op scores of our patient compared to the mean of the other pain regimens. The other studies did not have the same intervals as our patient to compare pain scores at every time interval. However we can see that at 60 minutes, our scores were not much different (3.8 and 4.0). And, our patient's pain scores at the immediate postop period, and from 2 hours post-op and onward remained below the mean score expressed by patients receiving other pain regimens.
Table 1: Postoperative VAS stratified by time interval and analgesic regimen. Mean pain scores were higher compared to those reported by the study subject at the two hour and twenty-four-hour timepoints. A higher score was reported in the first hour after surgery. View Table 1
With regard to postoperative opioid use (Figure 2), the specific opioid medications administered varied per study. In order to compare opioid use postoperatively, as depicted in Figure 2, all opioids were converted to intravenous morphine equivalents [12,13]. The average opioid consumption for the other studies is depicted by the dotted line. Notably, Meperidine alone had the highest 24-hour opioid consumption, and LAI alone had the lowest. Our patient had a 4-fold lower opioid consumption compared to the LAI alone.
Despite a low overall opioid consumption, and absence of adverse opioid related side effects, our patient did still require some opioid in the postoperative period. While the use of lidocaine infusions, esmolol, and ketamine may not completely prevent the use of opioids, acceptable pain control postoperatively can still be achieved and sustained without any of the opioid-related adverse effects. Therefore, it is reasonable to further study the use of lidocaine, esmolol, and ketamine as part of a multimodal, opioid sparing analgesic regimen in the perioperative period for patients undergoing laparoscopic cholecystectomy.
None.
None.
Jaclyn Altshuler helped contribute to the content and design of this manuscript.
Stephanie Ibekwe helped contribute to the content and design of this manuscript.