Acute respiratory failure is divided into four categories: hypoxemic (type I), hypercarbic (type II), perioperative (type III) and shock (type IV). We present a case of perioperative respiratory failure in a patient with iatrogenic phrenic nerve injury after mediastinoscopy and the implications for a high index of suspicion as well as appropriate diagnostics.
Anesthesia, Complications, Respiratory failure, Mediastinoscopy, Phrenic nerve injury
Of the four types of respiratory failure, perioperative (Type III) respiratory failure is often due to hypoxemia or hypercarbia (i.e. Type I or II). The main cause of this perioperative respiratory failure is the development of atelectasis. The volume of gas in the lung at which the small conducting airways remain patent at the end of tidal volume breathing is the closing volume. Closing capacity is comprised of closing volume and residual volume. If the closing capacity exceeds functional residual capacity (FRC) during normal tidal breathing, the dependent lung units collapse. Contributing factors include patient characteristics (e.g. chronic obstructive pulmonary disease, pre-existing pulmonary pathology, neuromuscular weakness, obesity), procedural complications (e.g. intraoperative recurrent laryngeal or phrenic nerve injury) and general anesthesia. Residual anesthetic, inadequate neuromuscular blockade reversal, or inadequate analgesia can also lead to type III respiratory failure. We present a case of an inadvertent intraoperative phrenic nerve injury posing a challenge in postoperative management.
A 76-year-old woman, BMI 23.8 kg/m2, American Society of Anesthesiologists (ASA) physical status 3 presented with symptoms of worsening shortness of breath, orthopnea and hoarseness. Her past medical history included breast cancer for which she underwent a left mastectomy, chemo- and radiation therapy five years prior to presentation. Additional history included asthma, hypertension, hypothyroidism, osteoporosis and a 30-pack-year history of tobacco smoking. Her medications were tamoxifen, albuterol, fluticasone, levothyroxine and alendronate.
Preoperatively, a physical exam revealed a respiratory rate of 24 breaths per minute and an oxygen saturation of 90% on room air (SpO2). She had decreased breath sounds bilaterally without wheezing or stridor. The remainder of her exam was unremarkable. Her preoperative testing included a normal complete blood count, coagulation and metabolic panels and electrocardiogram. Her room air arterial blood gas (ABG) revealed a pH of 7.44, PaCO2 36.3 mmHg, PaO2 56.4 mmHg and SaO2 90%. Her chest x-ray (CXR) showed a left mediastinal mass, hyperinflation of lungs, changes consistent with chronic obstructive pulmonary disease (COPD), a normal cardiac silhouette, extensive osteoporosis and thoracic degenerative disk disease. A chest MRI revealed a 4.5 cm mass in the aortopulmonary window, and possible involvement of the left recurrent laryngeal nerve (Figure 1, arrow).
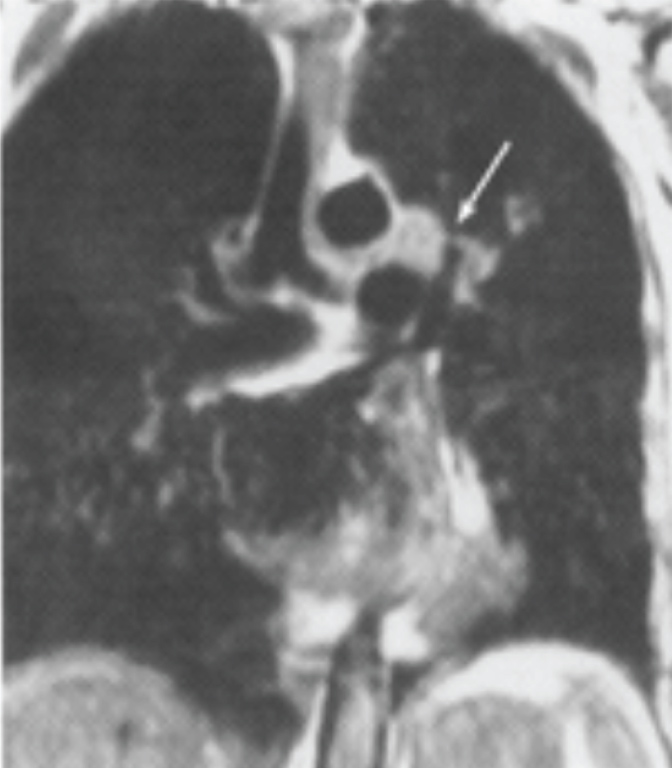 Figure 1: Patient MRI study demonstrating mass in the aortopulmonary window (arrow). View Figure 1
Figure 1: Patient MRI study demonstrating mass in the aortopulmonary window (arrow). View Figure 1
She subsequently underwent a bronchoscopy, left Chamberlain Procedure and a mediastinal mass biopsy for a preoperative diagnosis of mediastinal mass. Her operative course consisted of induction with intravenous lidocaine, etomidate, esmolol, fentanyl and vecuronium. The anterior mediastinoscopy was remarkable for a difficult dissection secondary to fibrotic tissue with minimal blood loss. The bronchoscopy, intercostal nerve blocks, chest tube placement and emergence/extubation were uneventful. Initial arrival to the post anesthesia care unit (PACU) revealed an alert and oriented woman who was able to follow commands and was pain free with SpO2 of 98% on 10 liters per minute via a face mask and a respiratory rate of 24 breaths per minute. All other vital signs were unremarkable. Within ten minutes, she was dyspneic and less arousable. She did not respond to jaw thrust, nasal airway insertion or naloxone administration. Breath sounds were diminished on the left with a functioning chest tube. Her ABG on 10 L/min of O2 via face mask revealed pH 7.11, paCO2 86 mmHg, paO2 223 mmHg and SaO2 99%. A chest X-ray now showed an elevated left hemidiaphragm, consistent with paralysis of the left diaphragm and possible injury to the left phrenic nerve (Figure 2). She was subsequently re-intubated.
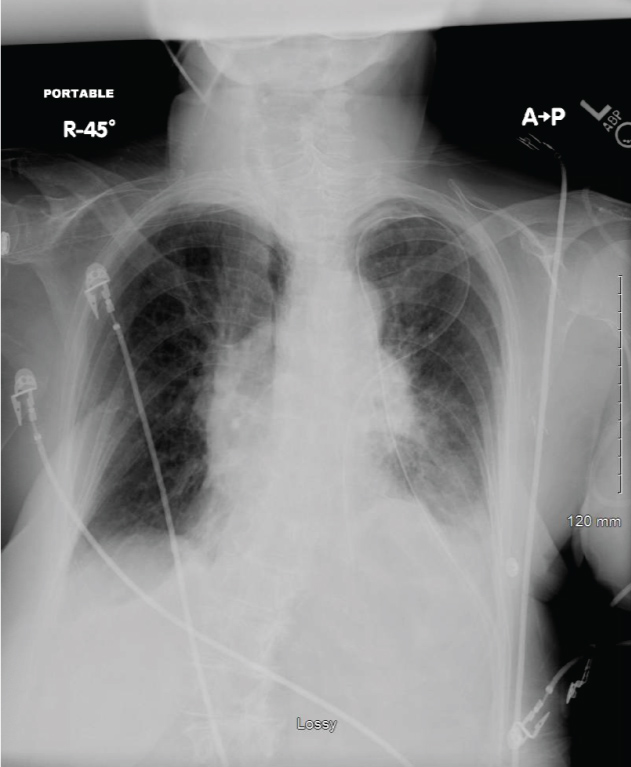 Figure 2: Patient chest radiograph demonstrating an elevated left hemidiaphragm. View Figure 2
Figure 2: Patient chest radiograph demonstrating an elevated left hemidiaphragm. View Figure 2
Iatrogenic phrenic nerve injury can lead to perioperative (Type III) respiratory failure that can manifest as early as in the PACU. For our patient, the left phrenic nerve was inadvertently damaged during a difficult surgical dissection for mediastinoscopy. Fibrotic tissue was abundant around the mass and thought to be due to her prior chest radiation therapy for breast cancer treatment.
In patients requiring mechanical ventilation secondary to acute respiratory failure, four main mechanisms have been described to encompass the relevant pathophysiology [1]. Type I is defined as the inability to maintain an arterial oxygen tension of greater than 60 mmHg. Common etiologies include those that result in an increased shunt fraction, such as pneumonia, pulmonary fibrosis, pulmonary edema and acute respiratory distress syndrome. Type II involves inadequate alveolar ventilation, such that hypercarbia ensues with an arterial CO2 tension of greater than 50 mmHg. Etiologies include those that originate from the CNS such as central hypoventilation, as well as myopathies, neuromuscular junction dysfunction, and airway obstruction. Type III involves respiratory failure during the perioperative period caused by factors associated with surgery and anesthesia, typically resulting in Type I and/or Type II failure. Type IV is a consequence of shock and hypoperfusion resulting in the need for mechanical ventilation to decrease the oxygen requirements of overworked respiratory muscles in the setting of decreased cardiac output.
The differential diagnosis for Type III respiratory failure in our patient was broad. Well known to our specialty is the inevitable reduction in functional residual capacity (FRC) intraoperatively and postoperatively--most often due to abnormal abdominal mechanics-to a point below the closing volume such that there is increased collapse of dependent portions of the lungs and atelectasis. Other common etiologies seen in the PACU include residual neuromuscular blockade, opioid effect, electrolyte imbalances, laryngospasm/bronchospasm, pulmonary edema secondary congestive heart failure, pneumothoraces, and in our case phrenic nerve paresis. Of significance, in an analysis of a quality assurance database conducted by P Lee, et al. [2] inadequate reversal of muscle relaxant was the cause of reintubation in only 5.8% of cases; however, electrolyte imbalance, age and comorbidities were not specifically studied. Such factors can alter pharmacodynamics and potentiate neuromuscular blockade resulting in a potential underreporting of the re-intubation rate [2]. Excessive opioid administration as a cause for re-intubation was only 4.7%. In our case, a documented train-of-four of four twitches was present at the end of the case and a full reversal dose was given. The patient did not have any comorbidity to suggest an altered response to muscle relaxants. Naloxone was given in the PACU without effect, and the patient did not show signs of obstruction or laryngospasm/bronchospasm in the PACU. A CXR was ordered immediately and revealed a new elevated left hemidiaphragm, indicating a possible acute injury to the left phrenic nerve.
Mediastinoscopy has a reported morbidity rate of 0.3% to 3.0%; included in that percentage is phrenic nerve injury, but also a multitude of other iatrogenic injuries (e.g. hemothorax, chylothorax, pneumothorax, esophageal or tracheal injury) secondary to the complex anatomy of the mediastinum [3]. Despite the relative low incidence of these complications, the anesthesiologist must remain cognizant of these potential adverse events to adequately care for the patient both intraoperatively and postoperatively.
It is important to note that the incidence of phrenic nerve injury is increased in certain types of surgical procedures. These include head and neck, cardiac, and thoracic surgery of the lungs and/or esophagus as well as the mediastinum [4]. The incidence of phrenic nerve injury following cardiac surgery historically was reported in the range of 24-73%, where its etiology was thought to be ice in the slush solution that bathed the heart, leading to a demyelination injury of the phrenic nerve. Modern technical modifications including an insulating pad to protect the phrenic nerves has decreased the incidence to 2-17% [5,6]. Lastly, there are non-surgical causes to phrenic nerve injury, including compression by mass effect, regional anesthesia (e.g. interscalene and supraclavicular blocks), blunt trauma, idiopathic phrenic neuropathy, post viral phrenic neuropathy, radiation therapy, cervical chiropractic manipulation, and birth injury.
Most patients with unilateral phrenic nerve injury or paralysis remain asymptomatic at rest [7]. Nonetheless, expansion of the rib cage becomes dependent on intercostal, scalene and sternocleidomastoid--i.e. the "accessory"--muscles. Upon reduction in pleural pressure during inspiration, the paralyzed diaphragm moves cephalad and the abdominal muscles inward. This reduction in ipsilateral lung ventilation, especially of the lower lobe, can lead to hypoventilation and hypoxemia especially in the debilitated, the obese, those patients with underlying lung pathology, and postoperative patients such as ours. Such physiological derangements may produce symptoms of dyspnea with exertion and orthopnea. Often these patients require sitting in the upright position, administration of supplemental oxygen, and/or institution of noninvasive or invasive mechanical ventilation to augment tidal volume as was seen in our case. Unilateral phrenic nerve injury reduces the total lung capacity (by 14 to 29%), forced vital capacity (by 23 to 27%), and inspiratory capacity (by 10 to 20%) compared with baseline or predicted parameters [8,9].
Although the most well known confirmatory test for phrenic nerve palsy is the "sniff-test" fluoroscopy that allows real-time evaluation of diaphragmatic movement, performing fluoroscopy in our patient with impending respiratory failure would have been impractical and unsafe; the diagnosis was presumptively made by chest x-ray findings in conjunction with associated intraoperative dissection difficulties. A chest x-ray revealing an elevated hemidiaphragm as in our case is sensitive for phrenic nerve injury; however, it is not specific. Complete phrenic nerve palsy may be diagnosed by paradoxical cephalad movement of the diaphragm or a 75% or greater reduction in diaphragmatic movement by ultrasound evaluation. Diaphragmatic ultrasound has been shown to have high sensitivity (93%) and specificity (100%) in diagnosing phrenic nerve dysfunction [10-12]. In retrospect, one could have quickly used an ultrasound to confirm diagnosis of phrenic nerve palsy at bedside in our patient. Nonetheless, fluoroscopy was ordered and performed in the intensive care unit (ICU) afterwards which confirmed left phrenic nerve paralysis.
Most patients with unilateral paralysis require no treatment, with good long term prognosis. Indeed, our patient was able to be discharged with no supplemental oxygen after extubation and a hospital stay of seven days. Patients with underlying pulmonary disease or bilateral paralysis may have a more complicated course, and prognosis is usually poor [13].
It remains difficult to predict the time from recovery to baseline after initial injury/paralysis [14,15]. Gayan-Ramirez, et al. [15] concluded that the type (uni- versus bilateral) and etiology of phrenic nerve injury did not influence functional respiratory recovery, and only 43% of patients had partial reversal of the effects of diaphragm paralysis at 1 year. Ultimately, patients who display severe dyspnea, need for prolonged mechanical ventilation, or failure to thrive may require further treatment. For refractory cases of unilateral paralysis, diaphragmatic plication is an option with excellent short- and long-term results in carefully selected patients [16]. In regards to bilateral paralysis, initial treatment involves noninvasive positive pressure ventilation, with refractory cases ultimately requiring intermittent or continuous mechanical ventilation via a tracheostomy.
Although our patient was transferred to the ICU after re-intubation, she was extubated the following day and was able to go home with no supplemental oxygen in 7 days. A follow up CXR revealed a persistent elevated left hemidiaphragm, but the patient was able to tolerate it well on room air with no dyspnea on exertion. A high index of suspicion is needed when caring for patients undergoing procedures that have an increased risk for phrenic nerve injury, and prompt diagnosis and intervention, if needed, are paramount.