Severe anaphylactic reactions to midazolam and Clindamycin are exceedingly rare. Thorough surveillance, early recognition, and prompt intervention for these reactions are imperative due to the widespread use of both of these medications in the surgical setting.
We present the case of a 79-year-old male who was set to undergo an elective incisional hernia repair and received midazolam and clindamycin preoperatively, which was immediately followed by anaphylactic shock leading to cardiac arrest. His hospital stay was complicated by flash pulmonary edema and respiratory distress. On postoperative day eight, the patient’s condition stabilized and was discharged.
The widespread use of midazolam and clindamycin by anesthesia providers may make it appear that both agents are very safe for common use however, we highlight the rare but potentially disastrous adverse reactions that may occur from using these medications.
Presented are the cardiovascular considerations of anaphylactic shock due to an unknown culprit and the appropriate interventions that took place to ensure the patient’s survival.
Anaphylactic shock, Cardiac arrest, Anesthesia, Adverse drug events, Critical care
Anaphylactic shock has previously been linked to stress induced cardiomyopathy and acute heart failure. The mechanism behind this phenomenon is not fully understood however, it is theorized that the rapid release of immunomodulators during anaphylaxis, decreases systemic blood pressure, compromising myocardial tissue blood supply and propagating myocardial cell dysfunction. The mechanism for this massive vasodilation is explained by the sudden release of histamine and leukotrienes from mast cells. These mediators induce production of nitric oxide (NO), which increases concentration of cGMP, a key mediator of smooth muscle relaxation [1]. In addition, the sudden surge of leukocyte chemokines in anaphylaxis has been implicated in coronary artery vasospasm, another possible mechanism for reduced cardiac blood supply [2]. The resulting combination of reduced venous return, coronary vascular insufficiency, and loss of blood volume through plasma leakage may lead to an inability to meet cardiac oxygen demands, eventually potentiating myocardial dysfunction [2].
It has also been postulated that the increased production of catecholamines in the case of distributive shock may lead to cardiovascular stunning as the mechanism of stress-induced cardiomyopathy [3]. In one case report, antibiotic induced anaphylactic shock in the intraoperative period resulted in acute anterior and lateral left ventricular wall dysfunction and hypokinesis, suggesting that this allergic reaction led to impairment of coronary vessels [3]. Potential anaphylactic reaction effects on cardiac function warrant heavy scrutiny, especially in patients with a history of cardiac issues.
Midazolam is a commonly used benzodiazepine medication administered prior to surgery that functions as an anxiolytic and amnesiac. It is an appealing medication to use because of the rapid onset, increased patient satisfaction for managing preoperative anxiety, and the generally safe pharmacologic profile [4]. Very few cases of immediate anaphylaxis to midazolam have been reported in the literature however, when this hypersensitivity reaction has been reported, midazolam use has led to severe hemodynamic instability that may have been potentially fatal without early recognition and intervention [5].
Clindamycin is a commonly used preoperative prophylactic antibiotic. It has similar propensity at preventing postoperative infections compared to cephalosporins. There is a degree of cross-reactivity allergic reactions between penicillin and cephalosporins in patients who have penicillin allergies. Therefore, patients with penicillin allergies often receive clindamycin as preoperative prophylaxis. Adverse effects and anaphylaxis pertaining to the use of clindamycin as an alternative antibiotic are very rare [6]. There are very few reported cases of immediate hypersensitivity reactions to clindamycin. Cases of both pre-induction and post-induction immediate anaphylactic reactions with severe cardiovascular instability to clindamycin have been reported, and one such case resulted in cardiac arrest [7].
Identifying anaphylactic reactions early is critical to preventing morbidity. Physical signs include hypotension, cardiovascular collapse, dysrhythmias, respiratory failure, bronchospasm, flushing, erythema, urticaria, and loss of consciousness, typically beginning within five minutes upon exposure to allergen, so quick intervention is vital [8]. Other potential diagnoses must be considered as well.
We present the case of a 79-year-old male with a past medical history of coronary artery disease (CAD), hyperlipidemia, hypertension, atrial fibrillation, and atrial flutter who suffered a cardiac arrest following preoperative administration of midazolam and clindamycin for an elective incisional hernia repair.
A nuclear medicine, cardiac perfusion, gated stress test was performed 16 months prior, revealing no evidence of scintigraphic inducible ischemia, no evidence of scarred myocardium, normal left ventricular size, normal left ventricular systolic function with an ejection fraction (EF) of 71%, normal right ventricular size and systolic function.
2 mg of midazolam was administered preoperatively for operative anxiety management. Immediately after, the patient was taken to the operating room where 900 mg clindamycin infusion was started. The choice to use clindamycin was indicated due to the patient-reported penicillin allergy. A few minutes after administration, the patient reported feeling warm, flushed, dizzy, and having tinnitus. His pallor changed from pink to pale and grey. Vital signs revealed severe hypotension of 69/29 mmHg, heart rate of 97 BPM, and SpO2 of 96%. About one minute later, he began foaming at the mouth and subsequently lost consciousness. Over the next few minutes, blood pressure decreased to 56/19 mmHg and heart rate was 85 BPM. EKG waveform demonstrated ventricular tachycardia. The patient was immediately ventilated with 100% O2 through bag valve mask and then emergently intubated using 30 mg rocuronium as a paralytic agent and 100 mg propofol. End-tidal CO2 values of near 40 mmHg were observed. The patient received several doses of epinephrine boluses and infusion totaling 2.2 mg. 200 mcg phenylephrine, 20 mg famotidine, 100 mg esmolol, and 333.5 mcg esmolol were given in IV infusion. Suspected anaphylaxis was treated with two doses of 50 mg diphenhydramine, two doses of 100 mg hydrocortisone, and an albuterol inhaler. Additionally, two, 18 gauge peripheral IV’s were placed in the right hand and left ankle as well as a double-lumen, right internal jugular, central venous line.
The patient went into pulseless electrical activity and CPR was initiated, which led to return of spontaneous circulation (ROSC). Subsequent arrest occurred, so a second round of CPR, along with one round of cardioversion resulted in ROSC. A 20 gauge arterial line was placed in the right radial artery. Arterial Blood Gas (ABG) results demonstrated severe, mixed, respiratory-metabolic acidosis with an extremely low pH, high lactate, high pCO 2 , high PO2, low bicarbonate, normal sodium, normal potassium, high chloride, high hemoglobin, and high hematocrit. A second ABG was sent several minutes later, revealing an improved but still low pH, low pCO 2 , high pO 2 , low bicarbonate, normal sodium, low potassium, normal hemoglobin, and normal hematocrit. 50 mEq sodium bicarbonate, 1000 mg calcium chloride, 20 mEq potassium chloride, and 2 g magnesium sulfate were administered. A third ABG was sent, demonstrating that arterial pH normalized as well as a normal pCO 2 , high pO 2 , normal bicarbonate, normal sodium, low potassium, high chloride, high lactate, normal hemoglobin, and normal hematocrit (Table 1).
Table 1: Arterial blood gas #1 demonstrated severe, mixed, respiratory-metabolic acidosis. Arterial blood gas #2 showed improving arterial pH and arterial blood gas #3 demonstrated resolution of the acute acid-base disturbance. View Table 1
Transesophageal echocardiography was performed intraoperatively (Figure 1), six hours postop (Figure 2), and five days postop (Figure 3). EKG performed intraoperatively following ROSC demonstrated sinus rhythm with a borderline repolarization abnormality and a prolonged QT interval of 444 ms and QTc of 451 ms. Notably, there was no ST segment elevation present.
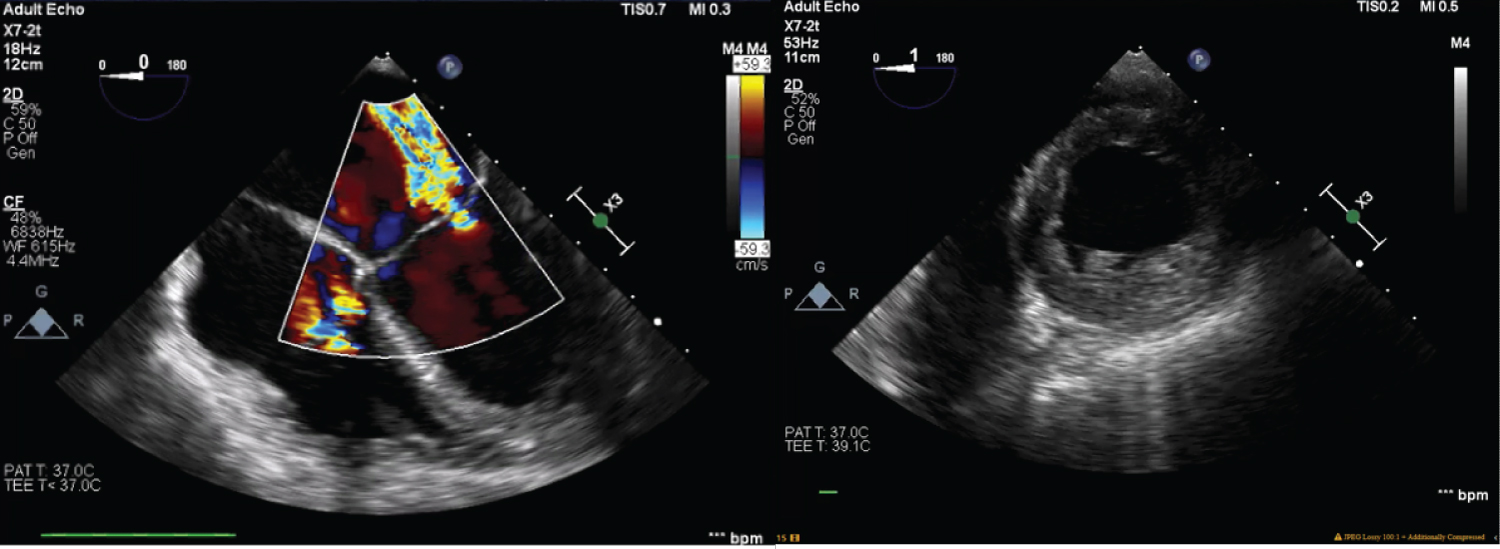 Figure 1: Transesophageal echocardiogram was performed intraoperatively, revealing 2+ mitral valve regurgitation, left ventricle ejection fraction of 25%, akinetic septum, and hypokinetic anterior and inferior left ventricular walls. There was also moderate aortic insufficiency.
View Figure 1
Figure 1: Transesophageal echocardiogram was performed intraoperatively, revealing 2+ mitral valve regurgitation, left ventricle ejection fraction of 25%, akinetic septum, and hypokinetic anterior and inferior left ventricular walls. There was also moderate aortic insufficiency.
View Figure 1
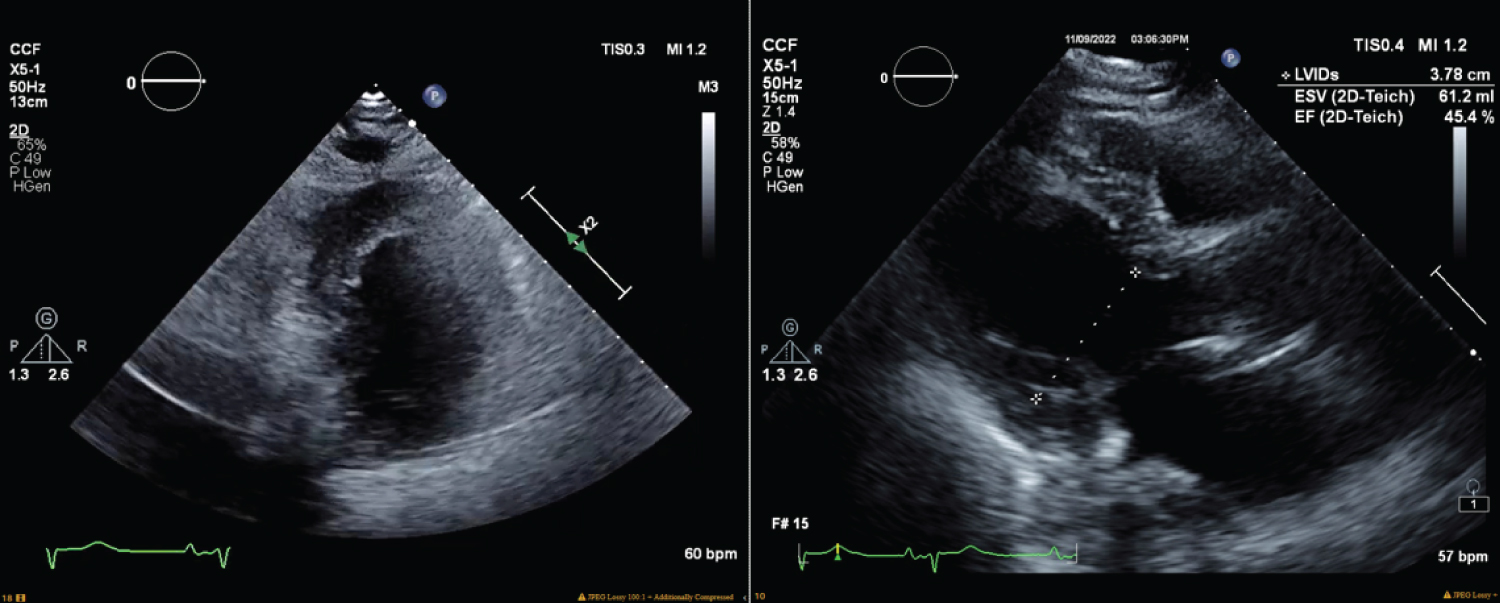 Figure 2: Transesophageal echocardiogram performed six hours after the cardiac arrest, revealed left ventricle ejection fraction of 45%, moderate right ventricular dilation, moderate right atrial dilation, and a mildly hypokinetic left ventricular wall, septum, apex, and inferior wall.
View Figure 2
Figure 2: Transesophageal echocardiogram performed six hours after the cardiac arrest, revealed left ventricle ejection fraction of 45%, moderate right ventricular dilation, moderate right atrial dilation, and a mildly hypokinetic left ventricular wall, septum, apex, and inferior wall.
View Figure 2
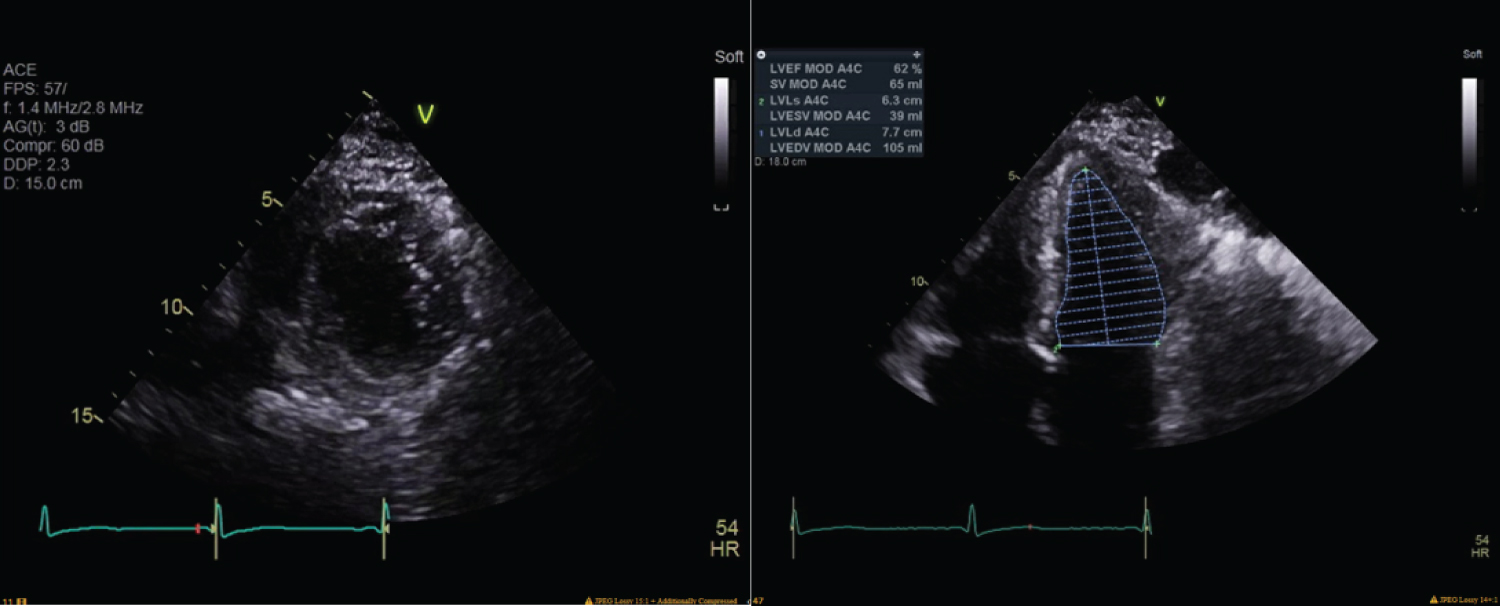 Figure 3: Echocardiography performed five days later revealed normal left ventricular size and mild hypertrophy, left ventricle ejection fraction of 64%, grade II left ventricular diastolic disfunction, moderate left atrial dilation, milt right atrial dilation, mild mitral regurgitation, moderate tricuspid valve regurgitation, mild aortic regurgitation, and an estimated right ventricular systolic pressure of 68 mmHg, consistent with moderately severe pulmonary hypertension.
View Figure 3
Figure 3: Echocardiography performed five days later revealed normal left ventricular size and mild hypertrophy, left ventricle ejection fraction of 64%, grade II left ventricular diastolic disfunction, moderate left atrial dilation, milt right atrial dilation, mild mitral regurgitation, moderate tricuspid valve regurgitation, mild aortic regurgitation, and an estimated right ventricular systolic pressure of 68 mmHg, consistent with moderately severe pulmonary hypertension.
View Figure 3
Following ROSC, the patient’s hemodynamics were stabilized to a BP of 106/53 mmHg, pulse of 55 BPM, and SpO 2 of 98% on 100% FiO 2 . He was then transferred to the SICU, manually ventilated with 100% O 2 . In the ICU, the patient was managed with fentanyl for pain control, propofol for sedation, telemetry monitoring, and epinephrine/norepinephrine for blood pressure support. Troponin levels intraoperatively were < 0.010 and shortly after arriving to the SICU rose to 0.273. Intraoperative CK and CK-MB were within normal ranges, with values of 71 and 2.4, respectively. Subsequent EKG in the ICU demonstrated a prolonged QT interval of 574 ms and QTc of 539 ms. Cardiology reported findings of stress cardiomyopathy, likely due to anaphylaxis. Further cardiac consultation during hospital stay confirmed atrial fibrillation with rapid ventricular response. Metoprolol for atrial fibrillation management and warfarin were started. The patient’s hospital course was complicated by flash pulmonary edema, acute decompensated heart failure, and respiratory distress, confirmed with chest X-ray. Subsequent diuresis with furosemide was initiated. Troponin began to decrease from 0.190 the evening of the cardiac arrest, to 0.118 and 0.082 the following day. Chest X-rays performed on the day of cardiac arrest and the next several days were only significant for mild atelectasis and pleural effusion.
The patient was successfully extubated after two days. A tryptase test taken at the time of the cardiac arrest was positive, with a reading of 16.2 ng/ml, compared to normal tryptase value of < 8.4 ng/ml, indicating a possible anaphylactic reaction to either clindamycin or midazolam. The immediate anaphylactic reaction to one of these medications likely led to the cardiac arrest and cardiomyopathy exhibited. Eight days following the cardiac arrest, the patient was discharged home in a stable condition.
The rapid cardiovascular collapse exhibited in this case had multiple differential diagnoses that had to be considered while performing life-saving interventions. Anaphylactic reaction seemed the most likely at the time. Other potential differentials were massive myocardial infarction (MI), flash pulmonary edema, massive pulmonary embolism (PE), air embolism, and others.
The patient’s history of CAD and atrial fibrillation make massive MI a plausible differential. MI potentially presents with some shared features such as shortness of breath, abnormal cardiac rhythm, dizziness [9], abnormal echocardiogram findings, and elevated troponin level. Evidence against an acute MI includes the lack of any acute ischemic changes on the EKG following ROSC. In addition, the patient had had a negative cardiac stress test previously. It is still important to consider the possibility of MI for sudden cardiac arrest.
Air embolisms can also precipitate acute cardiac arrest. The use of peripheral venous catheters makes it possible that air could get into venous circulation. However, if this had occurred, while the patient was intubated, there would have been a reduction in end-tidal CO 2 because of circulatory compromise to the perfusion of pulmonary arteries. Additionally, there would have been more specific EKG findings, such as right heart strain [10].
Massive PE is another cause of cardiac arrest that may have been possible in this scenario. When attempting to rule out PE in an acute setting such as this one, it is important to consider that PE induced cardiac arrest is associated with near-total right ventricular outlet obstruction. During cardiac arrest, echocardiogram images would have demonstrated right ventricular systolic dysfunction without having as much implication on the left side of the heart. Other expected findings with PE such as reduced oxygen saturation and right heart strain on EKG would have been seen.
Another differential diagnosis could be cardiac tamponade. The patient history of CAD makes a ventricular free wall rupture leading to fluid accumulation in the pericardial space a plausible explanation for the presentation. Cardiac tamponade may present similarly to this case. Fluid surrounding the heart prevents diastolic relaxation leading to hypotension and eventually, pulseless electrical activity. However, this was ruled out in this case due to the absence of visualized pericardial fluid during the intraoperative echocardiogram [11].
We should also consider malignant arrhythmias and pneumothorax however, these causes would have much more specific findings as part of the clinical picture than what was seen with this case. Preexisting structural heart disease such as severe valve abnormalities, coarctation of the aorta, and hypertrophic obstructive cardiomyopathy may also lead to rapid cardiac arrest however, there was no past medical history indicating these were present. With the cardiac history of the patient, these structural abnormalities would most likely have been previously discovered.
Tryptase is a marker for mast cell degranulation. Some studies have indicated that serum tryptase > 12.0 ng/l has a specificity of up to 89% for detecting anaphylactic reactions [12]. It is thought that tryptase is maximally elevated between 30 minutes and 2 hours from the start of the anaphylactic reaction [8]. In this case, the tryptase blood sample was taken within 30 minutes which may help us infer that the calculated tryptase would have been even higher had it been collected later. This, coupled with the clinical presentation of reported flushing and warmth as well as the sudden severe hypotension helps to elucidate that anaphylaxis to one of the medications given was the most likely culprit for cardiac arrest in this patient. Specific allergy testing must be done to confirm the offending agent.
A possible explanation for the complicated hospital stay is the presence of flash pulmonary edema. This is the rapid accumulation of fluid in the extravascular space of lung parenchyma. Pulmonary edema is an acute decompensated state that may occur due to cardiogenic or non-cardiogenic causes. Cardiogenic pulmonary edema may occur due to various cardiomyopathies including valvular dysfunction, MI, or arrhythmia. As a result, diastolic cardiac filling is compromised, allowing blood to back up along the pulmonary vessels, increasing hydrostatic pressure, and allowing fluid leakage around the lung parenchyma. Non-cardiogenic causes occur due to increased pulmonary vessel permeability, resulting in fluid leakage. This may be due to intra- or extrapulmonary causes such as pneumonia, sepsis, or inhalation injury [13]. Anaphylaxis as well as the use of epinephrine for treating anaphylaxis have both been linked to acute respiratory distress syndrome (ARDS) and flash pulmonary edema [14]. It has been postulated that inflammatory mediators released during anaphylaxis could cause lung damage, resulting in non-cardiogenic pulmonary edema and ARDS. Additionally, the use of exogenous epinephrine for anaphylaxis, stimulates adrenergic receptors, constricting peripheral vasculature, and increasing left ventricular pressure, causing blood to back up to pulmonary vessels, resulting in cardiogenic pulmonary edema [14]. The global cardiac hypokinesis visualized on echocardiogram, coupled with the use of large-dose IV epinephrine makes flash pulmonary edema a likely sequelae of cardiac arrest and anaphylactic shock. This may explain the complicated hospital course that was seen.
Advanced Care Life Support (ACLS) guidelines outline the protocol for treating anaphylactic shock before and after cardiac arrest. Prior to cardiac arrest, the guidelines stress the importance of early clinical recognition of anaphylaxis and discontinuation of the potential agent. Once anaphylaxis is recognized, suggested interventions are, high-flow oxygen, 0.1 mg IV epinephrine, aggressive crystalloid fluid resuscitation, 25-50 mg of IV or IM diphenhydramine, H2 receptor blockers, inhaled albuterol or ipratropium for suspected bronchospasm, and early high-dose IV corticosteroids. Following cardiac arrest, guidelines are similar except that high dose IV epinephrine is given at an increased frequency and the CPR algorithm begins [15].
Here we demonstrated the cardiovascular considerations of anaphylactic shock due to an unknown culprit. We also demonstrated the appropriate interventions that took place to ensure the survivability of a patient going through an unexpected emergency. This is a unique case because both midazolam and clindamycin severe adverse reactions are very rare. The widespread use midazolam and clindamycin by anesthesia providers may make it appear that both agents are very safe for common use however, we highlight the potential disastrous adverse reactions that may occur from using these medications. Thorough surveillance for signs of allergic reactions as well as prompt intervention when they do occur, is vital to optimizing safe conditions for operative anesthesia management.
No conflicts of interest.
No sources of funding.
No assistance from medical writing experts.