Successful implant osseointegration is dependent primarily upon adequate bone quantity and quality at the desired implant site. But often times the implant candidate, after proper clinical examination and evaluation of the patient's diagnostic information, lacks adequate bone quantity or volume for implant placement. Often times, the patient's has adequate height of bone but lacks bone width. The ability to predictably generate horizontal bone or bone width in preparation for dental implants, or guided bone regeneration (GBR) is an important and necessary procedure, in implant site preparation. Successful bone regeneration depends upon several factors. The first of which is to prevent soft tissue in growth into the bone graft material [1,2] . This can be achieved with a barrier membrane which can be resorbable or non-resorbable [3-5]. The second factor is the membrane must allow space for regeneration to occur. In other words, the membrane cannot collapse into the graft. And the third factor is that there must be no mobility of the graft [1,3,6]. In this particular case presentation, these factors or principals were achieved using a Cytoplast® titanium reinforced membrane which is rigid and tents the membrane to allow space for bone regeneration, not allowing the barrier membrane to collapse into the graft. The membranes in our case were also secured with bone tacks to prevent migration of the bone graft and the membrane. Tenting of the membrane by the titanium frame within the membrane and stabilization of the membrane with tacks provides the optimum potential for bone regeneration.
The patient is a 52-year-old female with a non-contributory medical history. The patient was missing teeth #s 7, 9 and 10 and she was interested in replacing these teeth with dental implants. A Cone Beam Computer Tomography (CBCT) of the maxillary arch was completed and reviewed with a clinical evaluation also (Figure 1, Figure 2, and Figure 3). Significant labial concavities and bone atrophy was noted and guided bone regeneration (GBR) was recommended using a d-PTFE, Cytoplast® titanium reinforced membrane (Osteogenics Biomedical, Lubbock, TX, USA).
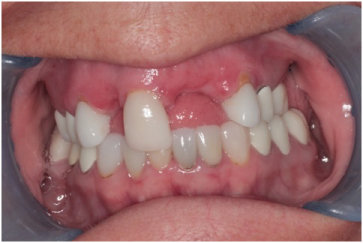 Figure 1: Labial concavity at the #s 7, 9, and 10 sites. View Figure 1
Figure 1: Labial concavity at the #s 7, 9, and 10 sites. View Figure 1
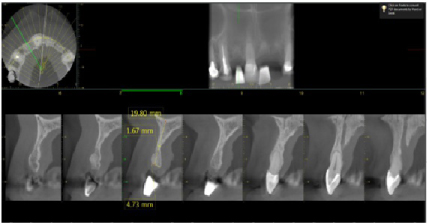 Figure 2: Pre-operative CBCT of the #7 site showing deficient labial bone. (1.67 mm of bone width). View Figure 2
Figure 2: Pre-operative CBCT of the #7 site showing deficient labial bone. (1.67 mm of bone width). View Figure 2
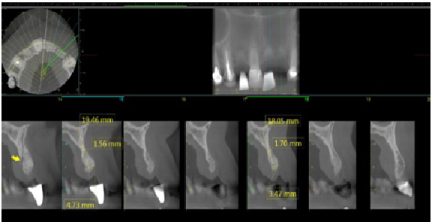 Figure 3: Pre-operative CBCT of the #s 9 and 10 sites showing deficient labial bone (less than 2 mm of bone width). The yellow arrow indicates the incisive canal, thus horizontal bone width is required for the subsequent implant to be placed and to avoid this vessel. View Figure 3
Figure 3: Pre-operative CBCT of the #s 9 and 10 sites showing deficient labial bone (less than 2 mm of bone width). The yellow arrow indicates the incisive canal, thus horizontal bone width is required for the subsequent implant to be placed and to avoid this vessel. View Figure 3
The patient received 2 gm of amoxicillin as an antibiotic prophylaxis (Zimox, Pfizer Inc., USA). Intravenous sedation was administered and approximately 30 cc of venous blood was drawn in order to prepare 3-5 cc of platelet rich fibrin (PRF). 2% Lidocaine HCL 1:100, 000 with epinephrine (Septodont Cook-Waite, Lancaster, PA, USA) was used for local infiltration at the #s 6-11 sites. Both surgical sites were prepared in the same manner. Bilateral papilla sparing incisions were completed (Figure 4 and Figure 5). Once the labial or buccal aspects were exposed, a #7011 bur was used to perforate the bone and establish bleeding bone (Figure 6 and Figure 7). At both sites, a Cytoplast® Ti-250 (Osteogenics Biomedical, Lubbock, TX, USA) titanium reinforced membrane shaped and sized to cover the proposed GBR sites and allow space for the bone regeneration to occur. The membrane each were secured with bone tacks first at the vestibule (Tru Tack® ACE™ Boston, MA, USA) (Figure 8). Then MinerOss® bone graft (Biohorizons, Birmingham, AL, USA) was placed at both surgical sites (Figures 9 and Figure 10) followed by a single superior membrane bone tack (Figures 11 and Figure 12). PRF was placed over the membranes (Figures 13 and Figure 14) and the surgical flaps were repositioned without tension with Cytoplast® and chromic sutures (Figures 15 and Figure 16).
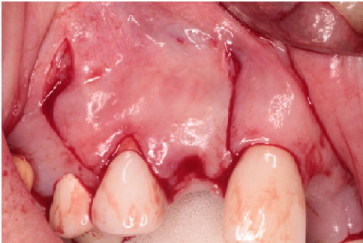 Figure 4: Flap design for the #7 site with papillae sparing releasing incisions. View Figure 4
Figure 4: Flap design for the #7 site with papillae sparing releasing incisions. View Figure 4
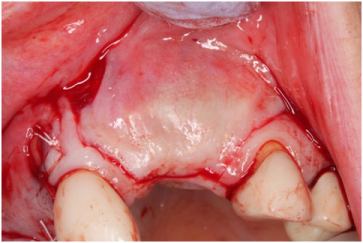 Figure 5: Flap design for the #s 9 and 10 sites. View Figure 5
Figure 5: Flap design for the #s 9 and 10 sites. View Figure 5
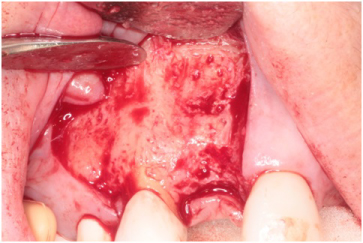 Figure 6: Bleeding bone established at the #7 site with a #701 bur. View Figure 6
Figure 6: Bleeding bone established at the #7 site with a #701 bur. View Figure 6
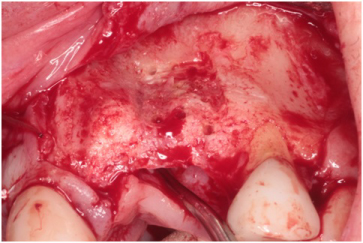 Figure 7: Site #s 9 and 10 bleeding bone established with a #701 bur. View Figure 7
Figure 7: Site #s 9 and 10 bleeding bone established with a #701 bur. View Figure 7
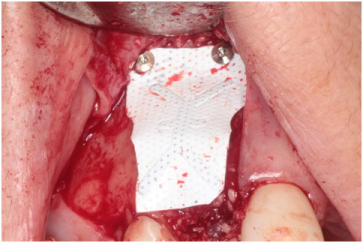 Figure 8: Two titanium tacks to secure the facial aspect of the titanium reinforced Cytoplast® Ti-250 membrane. View Figure 8
Figure 8: Two titanium tacks to secure the facial aspect of the titanium reinforced Cytoplast® Ti-250 membrane. View Figure 8
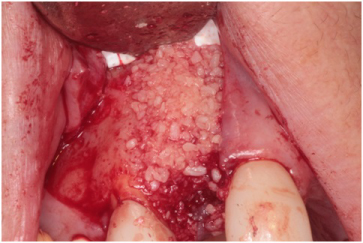 Figure 9: MinerOss® bone graft at the labial defect of #7 site. View Figure 9
Figure 9: MinerOss® bone graft at the labial defect of #7 site. View Figure 9
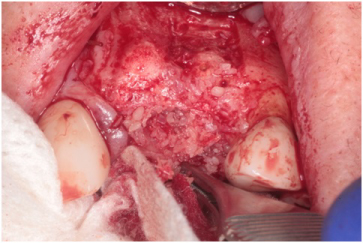 Figure 10: MinerOss® bone graft at the labial defect of #s 9 and 10 sites. View Figure 10
Figure 10: MinerOss® bone graft at the labial defect of #s 9 and 10 sites. View Figure 10
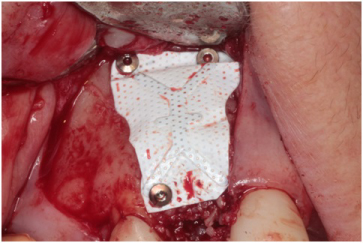 Figure 11: Superior aspect of the Cytoplast® Ti-250 membrane secure with a single tack. View Figure 11
Figure 11: Superior aspect of the Cytoplast® Ti-250 membrane secure with a single tack. View Figure 11
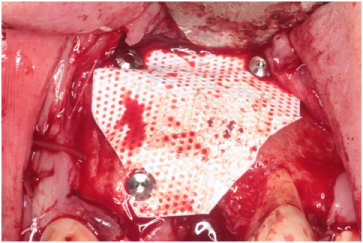 Figure 12: Two titanium tacks to secure the vestibular- facial aspect and a single tack superiorly securing the titanium reinforced Cytoplast® Ti-250 membrane. View Figure 12
Figure 12: Two titanium tacks to secure the vestibular- facial aspect and a single tack superiorly securing the titanium reinforced Cytoplast® Ti-250 membrane. View Figure 12
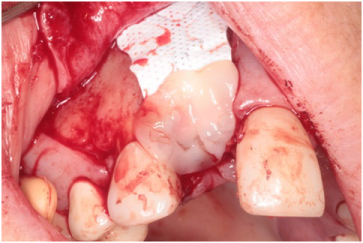 Figure 13: Platelet rich fibrin placed over the membrane. View Figure 13
Figure 13: Platelet rich fibrin placed over the membrane. View Figure 13
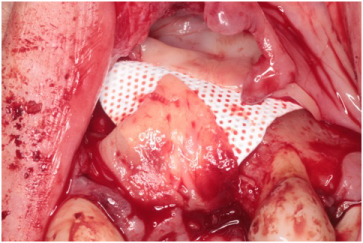 Figure 14: Platelet rich fibrin placed over membrane. View Figure 14
Figure 14: Platelet rich fibrin placed over membrane. View Figure 14
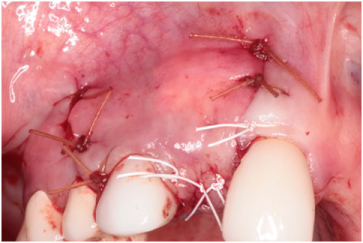 Figure 15: Flap repositioned without tension using Cytoplast® and chromic sutures at the #7 site. View Figure 15
Figure 15: Flap repositioned without tension using Cytoplast® and chromic sutures at the #7 site. View Figure 15
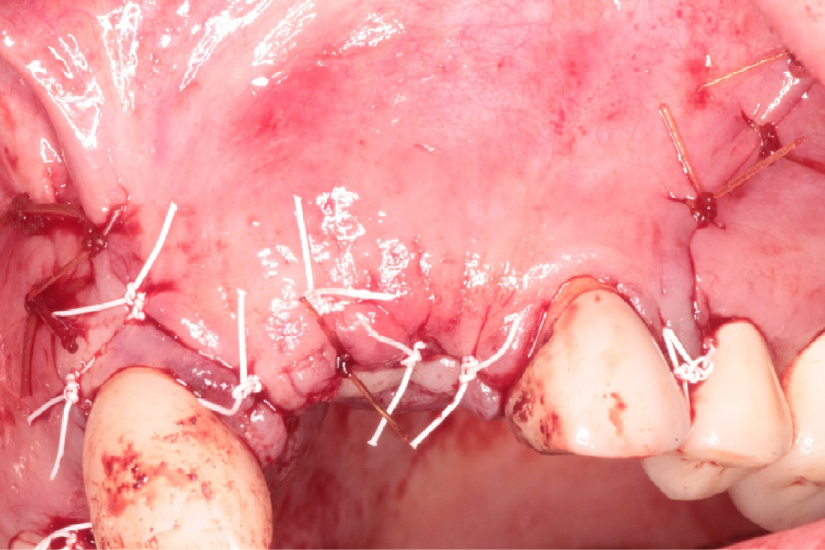 Figure 16: Flap repositioned without tension using Cytoplast® and chromic sutures at the #s 9 and 10 sites. View Figure 16
Figure 16: Flap repositioned without tension using Cytoplast® and chromic sutures at the #s 9 and 10 sites. View Figure 16
Four months later a new CBCT of the maxillary arch was completed and at all sites, #s 7. 9, and 10 with successful GBR achieved and implant placement was planned using the Anatomage™ (San Jose, CA, USA) implant planning software (Figures 17, Figure 18, and Figure 19). Each site went from less than 2 mm of width preoperatively to a width of 7 mm four month after grafting.
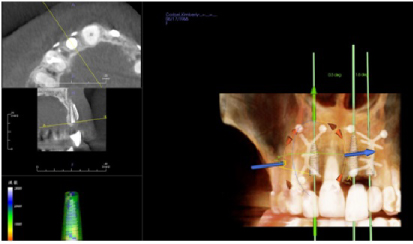 Figure 17: Post-op four months grafting #7 site CBCT and Anatomage™ implant planned. Notice the increased bone width from a preoperative width of less than 2 mm to a width greater than 6 mm. View Figure 17
Figure 17: Post-op four months grafting #7 site CBCT and Anatomage™ implant planned. Notice the increased bone width from a preoperative width of less than 2 mm to a width greater than 6 mm. View Figure 17
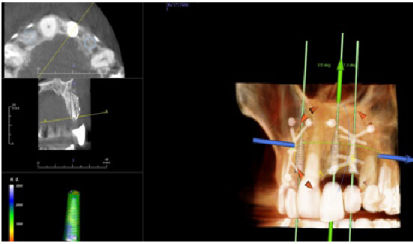 Figure 18: Post-op four months grafting #9 site CBCT and Anatomage™ implant planned. Notice the increased bone width from less than 2 mm to 7 mm. View Figure 18
Figure 18: Post-op four months grafting #9 site CBCT and Anatomage™ implant planned. Notice the increased bone width from less than 2 mm to 7 mm. View Figure 18
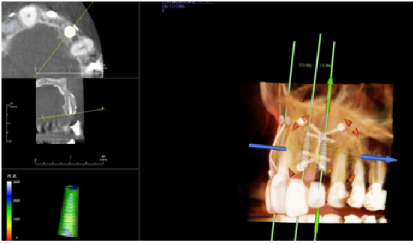 Figure 19:Post-op four months grafting #10 site CBCT and Anatomage™ implant planned. Notice the increased bone width from a preoperative width of less than 2 mm to a width after 4 months of 7 mm. View Figure 19
Figure 19:Post-op four months grafting #10 site CBCT and Anatomage™ implant planned. Notice the increased bone width from a preoperative width of less than 2 mm to a width after 4 months of 7 mm. View Figure 19
Using bilateral papillae sparing releasing incisions at surgical sites, the Cytoplast® membranes and tacks were removed and the implant sites were prepared showing excellent bone regeneration (Figures 20, Figure 21, and Figure 22). Standard protocol was used to prepare Straumann® Bone Level Tapered implants (Andover, MA, USA). (Figures 20 and Figure 22). Four months post implant placements with all 3 implants osseointegrated and provisional restorations in place. (Figures 23, Figure 24, and Figure 25).
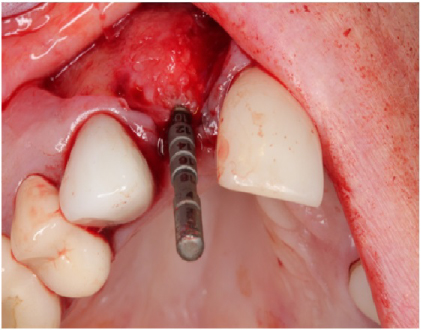 Figure 20: Implant site preparation for a 3.3 × 10 mm Straumann™ Bone Level Tapered implant. Depth gauge is in place. Notice the amount bone regeneration. View Figure 20
Figure 20: Implant site preparation for a 3.3 × 10 mm Straumann™ Bone Level Tapered implant. Depth gauge is in place. Notice the amount bone regeneration. View Figure 20
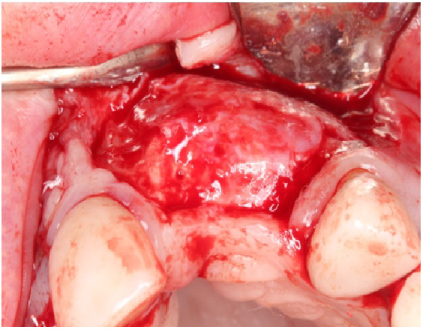 Figure 21: 4-months post-op guided bone regeneration at the #s 9 and 10 sites. Notice the bone regeneration compared to the pre-op condition. View Figure 21
Figure 21: 4-months post-op guided bone regeneration at the #s 9 and 10 sites. Notice the bone regeneration compared to the pre-op condition. View Figure 21
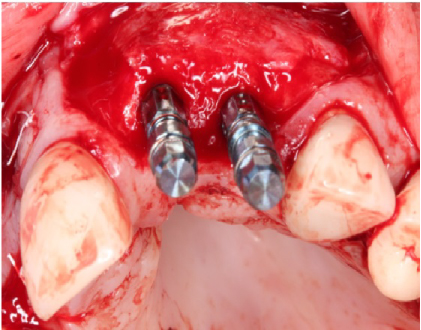 Figure 22: Straumann™ Bone Level Tapered implants placed at the #s 9 and 10 sites. View Figure 22
Figure 22: Straumann™ Bone Level Tapered implants placed at the #s 9 and 10 sites. View Figure 22
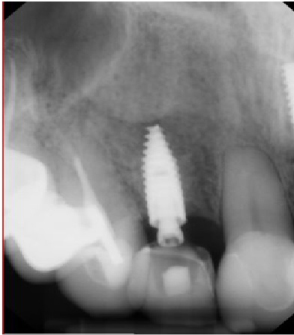 Figure 23: 4-month post-op implant placement #7, implant osseointegrated, provisional restoration in place. View Figure 23
Figure 23: 4-month post-op implant placement #7, implant osseointegrated, provisional restoration in place. View Figure 23
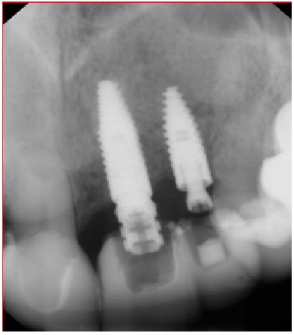 Figure 24: 4-month post-op implant placement #9, implant osseointegrated, provisional restoration in place. View Figure 24
Figure 24: 4-month post-op implant placement #9, implant osseointegrated, provisional restoration in place. View Figure 24
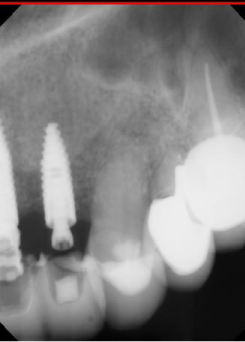 Figure 25: 4-month post-op implant placement #10, implant osseointegrated, provisional restoration in place. View Figure 25
Figure 25: 4-month post-op implant placement #10, implant osseointegrated, provisional restoration in place. View Figure 25
The use of guided bone regeneration to increase horizontal width in preparation for placement of dental implants is presented in the literature [1-4,6]. The barrier membranes used for guided bone regeneration include two categories either resorbable or non-resorbable, with the non-resorbable membranes being pure titanium mesh or titanium reinforced membranes [5,7-12]. Premature exposure of the titanium has been noted [5,10].
The obvious advantage of a resorbable membrane is that the membrane is resorbed by the body and there is no additional surgery to remove the membrane [5]. Disadvantages include the exact amount of time it takes for the membrane to resorb is not predictable, and for large grafts exposure leads to rapid degradation of the membrane [11].
In our particular case using a d-PTFE with an imbedded titanium frame, we had the advantage of a d-PTFE membrane and a titanium membrane. Exposure of the d-PTFE does not lead to infection or degradation of the membrane [4,8]. The embedded titanium frame allows for tenting of the membrane over the bone graft creating space for GBR [9-12]. What is unique about this case presentation are two factors. The first is that the titanium reinforced membrane was stabilized with tacks which are not always done with Cytoplast membranes. Because the membrane is a d-PTFE and non-resorbable, primary closure is usually not required or achieved. The membrane is easily pulled out like a suture in 4-weeks after grafting. The second factor with this case presentation that contributed to its success is that PRF was placed over the titanium reinforced membranes preventing membrane and titanium exposure and enhancing soft tissue healing. Titanium or titanium mesh exposure has been noted previously [5,10].