Viruses invade the cell through a highly selective mechanism known as "Receptor-Mediated Endocytosis". The virus's interaction with the target cell occurs via the formation of a vesicle that acts as a means of transport into the cell. Once internalized, the vesicle will originate an acidic organelle, containing digestive enzymes, called an endosome.
As it acts directly on the endosome, chloroquine (HCQ) may become the most important drug in the history of medicine when we talk about therapy for intracellular infections by viruses, bacteria, fungi and protozoa [1]. It changes the pH of the endosome created when the virus enters the cell. This change in pH acid to alkaline will be caused by chloroquine making viral replication unfeasible.
Here we try to show that HCQ will be the most important antiviral in history due to its broad spectrum of action.
It doesn't just act against a single virus like the so-called "protease inhibitors" used against HIV. It acts directly on the entry of any intracellular microorganism that tries to parasitize a cell. The Chloroquine is an alkaline drug, group of alkaloids, which dramatically alters the pH within the endosomes.
A small and quick literature review shows that, since the 1990s, virologists have already researched and confirmed the importance of the action of pH in the initiation of the cellular infection process [2].
So, in March 2020, forward to speculation suggesting the efficacy of HCQ in the fight against coronavirus SARS-2, our scientific curiosity led us to search the literature works to confirm its extraordinary action as an antiviral drug and its possible use in the treatment and in prophylaxis of viral diseases [3].
Kampmann and colleagues [4], working with the Togavirus Semliki Forest Virus (SFV), indicated an important and essential activity of the specific and conserved amino acid Histidine, in the fusion of the cell membrane of endosomes with the external viral protein. Exactly the one that recognized the target cell's receptor. They suggested that this Histidine was directly related to the release of the virus in the cytoplasm and its consequent replication. If this Histidine amino acid was changed into that viral protein that interacted with the cell receptor, this virus lost its ability to replicate. In other words, had disappeared!
Since the 90's, with DNA sequencing techniques, the literature already showed us that all Dengue viruses, flavivírus family, kept four Histidines conserved in the envelope (env) protein. All remained paired in the same position when a sequence alignment of the four serotypes was performed.
It was later confirmed that this characteristic occurred not only in dengue viruses but in all viruses that are members of the Flaviviridae family. In Figure 1 we highlight part of the envelope sequence of different flaviviruses.
 Figure 1: Sequences obtained from the Genebank of the flavivirus family: JEV (Japanese Encephalitis Virus); WNV (West Nile Virus); SLEV (Saint Louis Encephalitis Virus), Den 1 (Dengue 1); Den 2 (Dengue 2) and YEV (Yellow Fever Virus). The four Hisdines, derived from an evolutionary process, are indicated.
View Figure 1
Figure 1: Sequences obtained from the Genebank of the flavivirus family: JEV (Japanese Encephalitis Virus); WNV (West Nile Virus); SLEV (Saint Louis Encephalitis Virus), Den 1 (Dengue 1); Den 2 (Dengue 2) and YEV (Yellow Fever Virus). The four Hisdines, derived from an evolutionary process, are indicated.
View Figure 1
Supported in our scientific curiosity we decided to analyze the sequence of amino acids of other virus families to see if this feature was exclusive of the Flavivirus family. We were surprised to note that different families of viruses analyzed stood out several histidines kept in the outer protein of all members. Exactly in that protein responsible for recognizing the target cell receptor. Here, we also confirmed the presence of histidines, evolutionarily conserved. And most importantly, occurred in the same position when was done pairing d the Filoviruses, Togavirus, and Picornavirus, for the protein which binds to the cellular receptor (data not shown).
This phenomenon, undoubtedly evolutionary, which preserved histidines, could not be occurring by mere chance of nature. There had to be an explanation for this and we came up with a very viable and interesting hypothesis about it.
The histidines evolutionarily conserved in all virus families would be responsible for the escape of the virus enzyme digestion within the endosomes, at the very moment that this occurrence would acidification by the lysosome. Lysosomes are small cell organelles containing digestive enzymes and an acidic pH close to 5.
We had found a flaw in an evolutionary process and invasive occurred to hundreds of millions of years, involving the parasitic relationship between viruses and cells.
The protonation will of histidines at pH acid would be the "Achilles heel" of the virus. Maybe from all other intracellular microorganisms that invaded cells via receptor-mediated endocytosis.
We believe that a good sequencing and alignment study will show that the same should happen with other intracellular parasites. Which will explain the range of action of Chloroquine.
Any drug alkaline that has a tropism for the acid environment of the lysosome and endosome organelles, would completely stop the invasive process. And here we identify this property in Chloroquine and its analog Hidroxychloroquine. The invader would be digested before it escaped into the cytoplasm to start replication!
Since the laboratory demonstration of its efficient antiviral and antiparasitic action against several intracellular microorganisms [1] shown in the excellent review by Raoult, Chloroquine has been redirected in the search for the treatment of a series of infections, including HIV, systemic lupus erythematous, Q fever, and rheumatoid arthritis. However, it appears that other interests have been blocking access to this medication, even though she could eliminate the suffering and the deaths from diseases caused by viruses.
For us, it was evident that Chloroquine could stop or even prevent infection by any virus due to its alkaline characteristic. The only prerequisite would be to use it immediately, at the first sign of viral infection, exactly within the first five days of onset. Mainly because we know that viruses that cause diseases are still not recognized by the arsenal of the immune response in the first three days of infection. During this period, the virus multiplies freely, and vaccine or drug prophylaxis is indicated.
Considering the high rate of virus replication, any delay in medication would be disastrous. It's no use trying to treat an acute virus after a week of onset of symptoms. The exception would be for immunocompromised patients because, in this case, the replication viral can spend 30 days. For this reason, we suggest that hidroxichloroquine be a licensed drug that is easily accessible to everyone.
Chloroquine is a drug from the alkaloid family. It was already known in Europe since the 15th century when Spanish Jesuits visited the Peruvian Amazon. Like any alkaloid, it has a very fast and efficient absorption. After absorption, it will be located in organelles with acidic pH as it happens in lysosomes and endosomes. Having a very long half-life allows for prophylactic use and treatment for malaria. And here we're going to include all the intracellular parasitic microorganisms that use cell receptors to invade the cell.
In the presence of chloroquine, the pH of these small cell organelles, normally acidic pH5, will reach a pH close to 7. This change will prevent the essential acidic protonation of conserved histidines, making it impossible for the virus to expose the hydrophobic fusion domain and escape from the digestive vesicle. So capsid release and virus replication in the cell cytoplasm will not occur. As a consequence, the enzymatic digestion of the virus will occur and that ingested protein will follow its expected path, which would be a food source (Figure 2A and Figure 2B).
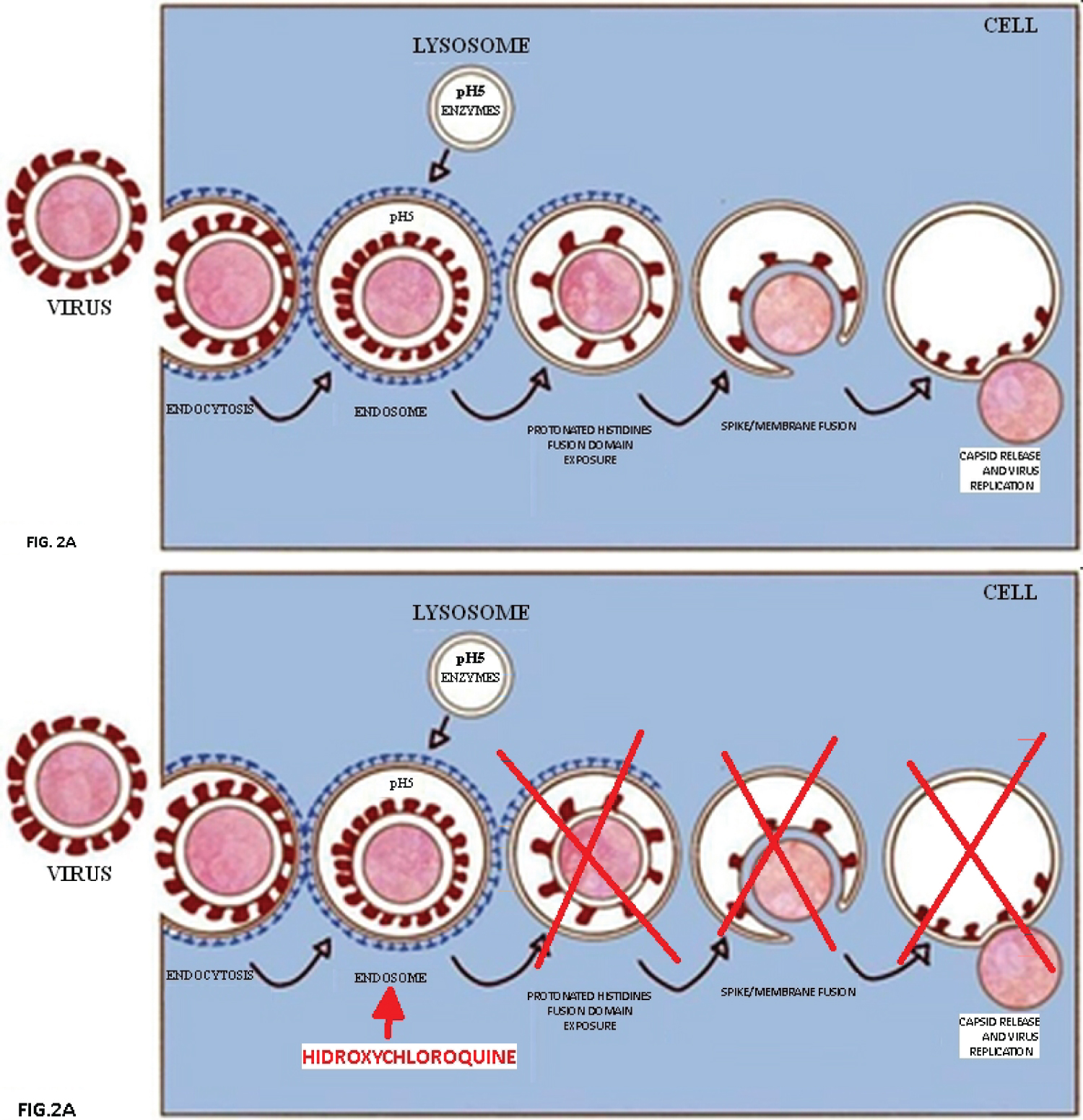 Figure 2: Prevention the essential acidic protonation of conserved histidines.
View Figure 2
Figure 2: Prevention the essential acidic protonation of conserved histidines.
View Figure 2
Faced with the discovery of the importance of the amino acid Histidine, we believe that if the protein of other intracellular parasitic microorganisms is sequenced, we will find the same mechanism of action. This would explain the already known capacity of Chloroquine in the prophylaxis of malaria and others.
Thus, a drug that could interrupt this evolutionary invasion process could be used against all those microorganisms that invade a cell through this pathway known as the "Trojan Horse".
We have now no longer doubt that chloroquine, one protons alkali receptor which diffuses passively through cell membranes going was concentrated in acidic organelles, would be an ideal tool to fight against such intracellular parasites. Its use would have, as a consequence, the neutralization of acidity, essential for activating the evolutionary histidines.
Chlorochine and the viral invasion: An evolutionary process that took place millions of years ago but due to the resistance of the scientific and medical community, we think it is important to clarify:
Given the mechanism described here and the attraction of chloroquine to acidic cell compartments, it is clear that chloroquine is not just an antimalarial or an intracellular antiparasitic. Due to its action linked to evolution, we believe that we will still have many related medicinal discoveries.
Chloroquine simply covers a fragile evolutionary barrier that cells presented, and intracellular parasitic microorganisms learned how to overcome the cell membrane barrier.
A wonderful drug, discovered centuries ago in the Amazon region by native Indians, could become the most important medicine in the history of humanity in combating viruses. Its alkaline characteristic, promoting the alkalization of the endosome, becomes disastrous for intracellular microorganisms.
Our studies indicate that the bases for explaining the action of chloroquine go back hundreds of millions of years of evolution. There is no denying its therapeutic use in viruses and other intracellular microorganisms.