Programmed death-1 (PD-1) is major inhibitory molecule that reduces T cell activation. PD-1 and their polymorphisms have separately been shown to be associated with hepatitis B virus (HBV) infection. Therefore, we aimed to investigate the association of PD1 polymorphisms with potential of chronic hepatitis B infection in Iranian population to do a Case-Control study.
In this study HBV DNA, and PD-1 mRNA, were extracted from 60 patients with chronic hepatitis B and 60 healthy blood donors as control. HBV Viral Load, polymorphism genotype of the two groups and level expression of PD1 gene were evaluated using Real time PCR method.
Age mean of the control group was 40.7 ± 3.7 and 35.86 ± 11.18 year's old age for the case group. The GG allele between the two groups had the same frequency (73.3% in CHB group and 80% in healthy group). The GA allele in patients with hepatitis B (20%) was most frequent. There was a significant association between the GA allele and chronic hepatitis B infection. There was no significant relationship between the ages of the subjects with SNPrs10204525. Also, the expression of PD-1 mRNA was higher in people with hepatitis B than in healthy group.
The results of this study showed that there is a significant relationship between the presence of rs10204525 polymorphisms in the PD-1 gene in patients with chronic hepatitis B infection and the association between the GA allele and chronic hepatitis B can be linked.
Chronic hepatitis B, Single nucleotide polymorphism, rs10204525, Programmed cell death-1
The viral infections cause many deaths in many parts of the world. Hepatitis B virus (HBV) infection is also one of those viral diseases and a major public health problem [1]. HBV can cause a variety of diseases in humans from inactive carriers to progressive liver disease, including chronic hepatitis B, cirrhosis and hepatocellular carcinoma (HCC) [2]. More than 350 million people in the world are chronic hepatitis B which is caused by infection with the hepatitis B virus. Most people infected with hepatitis B virus are able to remove the virus but in 5 to 10 percent of the population, the disease progresses to chronic disease [3]. Chronic hepatitis B infection refers to an inflammation of the liver that lasts at least 6 months, and the person infected with hepatitis B virus and HBs Ag remains positive up to 6 months post-infection [4]. The immune system is main cause of chronic hepatitis. An individual's immune system clears the HBV virus during an acute infection and, as a result, improves the disease when fully functioning correctly, so in an immune defect, acute infection becomes a chronic infection [5]. Several studies have shown that the host genetic context, including the diversity of genes involved in the immune system and cytokines, and single nucleotide polymorphisms, have a significant role in determining the immunogenicity and clinical course of the hepatitis B virus infection [6]. Cytotoxic T-lymphocytes (CTLs) play a major role in the fight against acute hepatitis B infection and remove of viral infection from hepatocytes. However, if the virus does not get properly cleaned out of the body, the hepatitis B virus remains, and a dynamic balance between virus replication in hepatocytes and the host's immune response is established and, in the long run, causes the progress of the disease [7]. In fact, in patients with chronic hepatitis B infection, viral T-specific cells did not have high activity, which would result in the production and secretion of adequate antiviral cytokines, inappropriate functioning of cytotoxic T lymphocytes, and thus the persistence of the virus in the body [8]. The Programmed cell death-1 (PD-1, CD 279) is a 55-KD protein and a type of the immunoglobulin super family and is an immunological receptor belonging to the CD28/CTLA4 family and is known to be an inhibitor of receptor [7,9]. PD-1 presented at the level of activated T cells, B lymphocytes, natural killer cells and myeloid dendritic cells, and can be seen to a high degree level at T lymphocytes that enters to the resting stage [10,11]. The inhibitory signals provided by PD-1 and its ligands, PD-L1 and PD-L2 both of which are located in 9p24 chromosome location, are necessary to maintain the balance of T-cell activation, tolerance and immune-mediated tissue damage, and inhibition of its function can result in T cells returning and their effective re-activity [12-14]. The PD-1 gene is located on chromosome number 2 in the q37.3 region, and more than 30 single nucleotide polymorphism sites (SNPs) are in different regions of the gene include promoter, intron, exon, and 3'UTR region [15-17]. Studies have shown that there is a link between these SNPs and various diseases. In recent studies, an SNP in the 3' UTR region of PD-1 gene, rs10204525 has been shown to be associated with the pathogenesis of chronic hepatitis B infection [11,18,19]. However, there is still not much information about this SNP and its association with chronic hepatitis B, but it seems the importance of PD-1 in regulating activity of T cells and its role in the development of chronic viral infections; Accordingly, we design a case control study for evaluation the level of PD-1 mRNA expression in peripheral blood nuclear cells and its relation with clinical parameters and correlation between polymorphism of PD-1 (rs10204525) and susceptibility of chronic hepatitis in patients with HBV infection in Kerman province, southeast of Iran.
In a case control study 60 blood sample were collected from patients with chronic Hepatitis B virus infection and 60 samples were collected from healthy blood donors as control group during July 2016 to October 2017. Mean years old age of case group was 40.7 ± 3.7, and for control group was 35.86 ± 11.18 years.
About five ml of peripheral blood were collected from each patient into EDTA-containing vacutainer tubes. Plasma was separated and stored at -70 ℃. HBV DNA was extracted from 200 µL of plasma with High Pure Viral Nucleic Acid kit (Roche Diagnostics, Germany). Extracted DNA pellets were resuspended in 100 µL of prewarmed Elution buffer and stored at -20 ℃ until use. For Quantification of HBV DNA we use Real Time HBV kit (artus HBV kit, Qiagen, Germany). Quantitative determination of the amplified products was done with the Rotor Gene Q (Qiagen, Germany).
Detection of rs10204525 was done using Real Time TaqMan probe assay with specific primers and probes from position 251 of the mRNA PD-1 gene (NM_005018) that designed by Beacon designer software (Primer Biosoft, USA). Sequences of primers and probes were shown in Table 1. Primers and probes synthesized by Metabion Company (Metabion international AG, Germany). For Real Time PCR and detection polymorphisms of rs10204525, Five µl of DNA sample that was extracted from samples, combined with 15 µl reaction mixture of TaqMan Master Mix (Thermo fisher Scientific, USA) contain 10 pmol of each primer and five pmol of probes. The condition for the TaqMan assay was initially 10 min with hot start Taq DNA polymerase at 95 ℃ followed by 40 cycles at 95 ℃ for 5 sec and 60 ℃ for 40 sec. In each cycle at the extension step, the fluorescent Probe signals of rs10204525 were measured, at Green channel for G allele (FAM), Yellow channel for A allele (JOE). Quantitative determination of the amplified products was done with the Rotor Gene Qthermo cycler (Qiagen, Inc).
Table 1: Primer and probe sequences for detection of SNP and PD-1 gene expression. View Table 1
RNA was extracted from a 100 µl volume of serum sample using precipitation method (RIBO-prep, ILS). Briefly, specimen was added to a tube containing 300 µl of lysis buffer and 400 µl of precipitate buffer, mixed, and incubated for 10 min at room temperature; and centrifuge in 13000 rpm for three minutes. Then washed once with 500 µl of wash buffer W3, once with 200 µl of 70% (V/V) ethanol (Wash buffer W4), and then dried at 60 ℃ for 10 min. It was then resuspended in 50 µl of RNase-free water and converted into cDNA by RT-PCR. For RT 5 µl of RNA was added to a reaction mixture (15 µl) containing 20 mM Tris-Hcl (pH 8.4), 50 mM KCl, 7.5 mM MgCl2, each deoxynucleoside triphosphate at a concentration of 1.5 mM, 25 ng of each random primer [(pdN)6;, 1.6 U of RNasin, and 200 U of Moloney murine leukemia virus reverse transcriptase (Thermofisher, Germany). The reaction mixture was incubated at room temperature for 10 min, 37 ℃ for 45 min, and 95 ℃ for 5 min and quenched on ice.
For determination expression level of PD1 gene, after Synthesis of cDNA, add five microliters of cDNA in duplicate into the optical 0.1 µL microtube. The Maxima SYBR Green Master mix (Thermo fisher) were used base on instruction kit. After alignment of these regions between all of them in EBML-EBI, Real time PCR primers were design for target mRNA gene (NCBI Reference Sequence: NM_005018.2), also; GAPDH gene were used for internal control. All of sequences of primers were purchased from Metabion Company (Metabion international AG, Germany) (Table 1). Real-time PCR was carried out on an Rotor GeneQ Qthermo cycler (Qiagen, Inc.) using the following conditions: 95 ℃ for 10 min, followed by 35 cycles of 95 ℃ for 15 sec and 60 ℃ for 30 sec. Raw data can then be analyzed with REST Relative Quantification Software version 2.2.3 (Qiagen, Inc.), generally using the automatic cycle threshold (Ct) setting for assigning baseline and threshold for Ct determination. Delta Ct values were used for the analysis. Relative expression (RE) of the sample gene was calculated using the ΔΔCT method and, also we used GAPDH for internal control or housekeeping gene.
The serum blood from the patients with chronic hepatitis B was assayed for Serum Glutamic-Oxaloacetic Transaminase (SGOT), Serum Glutamate-Pyruvate Transaminase (SGPT) and alkaline phosphatase (ALK) at our clinical laboratory using an automated analyzer (Hitachi, Cobaas). HBsAg and HBeAg as well as Anti-HCV , Anti-HIV and Hepatitis D antigen were determined using commercial Enzyme-linked Immunosorbent Assay (ELISA) technique kits (Diapro, Italy).
Out of 60 samples of case group were 43(71.6%) Males and 17(28.4%) females and in control group were 56(93.3%) Males and 4(6.7%) females. In Table 2, the relationship between SNPrs10204525 and the gender of people in different groups has been investigated. Regarding the results, there was no significant relation between sex and SNP rs 10204525. Polymorphism of the rs10204525 has a significant difference in hepatitis B and healthy subjects (P.Value < 0.001). So percent and prevalence of GG allele is equal in healthy and chronic hepatitis B patient groups, but frequency of AA allele in healthy people and GA allele in chronic hepatitis B patient group were more than other groups. In other words, there is a significant relation between the GA allele and the hepatitis B infection (Table 3). The relative mRNA expression of PD-1 in patients with chronic hepatitis B, HBeAg positive, HBe Ag negative and healthy group was shown in Figure 1. The expression level of mRNA PD-1 in HBeAg positive patients is higher than HBe Ag negative and in patients with chronic hepatitis B, is more than, HBe Ag negative group. All of 120 samples in this study (case, control), 8 samples had HBeAg positive and 112 samples were HBe Ag negative. There was no significant relation between HBe Ag and SNP alleles of PD-1, (P > 0.33) (Figure 2). SNP alleles of rs10204525 have been evaluated in chronic hepatitis B with HBV DNA load. According to the results, there is a significant relationship between rs10204525 polymorphism with HBe Ag (P < 0.05). So that in HBe Ag positive patients, the GG allele was more than other alleles and the AA allele were least of alleles. Regarding the results, the female or male status of the subjects in the study groups is not related to SNPrs10204525 (Table 3). The levels of Serum Glutamic-Oxaloacetic Transaminase (SGOT), Serum Glutamate-Pyruvate Transaminase (SGPT) and alkaline phosphatase (Alk) were measured. The results showed that mean of SGOT was 39.20 ± 3.96 IU/ml, Alk value of 107.63 ± 10.01 and SGPT was 40.98 ± 4.91. There was a significant relation between SGOT, SGPT and GG allele but no about Alk (Data not shown). Investigating the relationship between SNPrs10204525 and chronic hepatitis B with HBV DNA in different groups showed a significant relationship between SNPrs10204525 and HBV DNA load in hepatitis B virus (P < 0.05) (Table 4). In patients with chronic hepatitis B, the rate of the GG allele is more than other salleles.
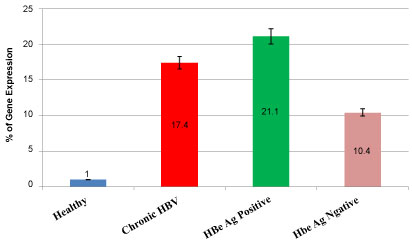 Figure 1: Relative gene expression of PD1 mRNA in three groups of Patients.
View Figure 1
Figure 1: Relative gene expression of PD1 mRNA in three groups of Patients.
View Figure 1
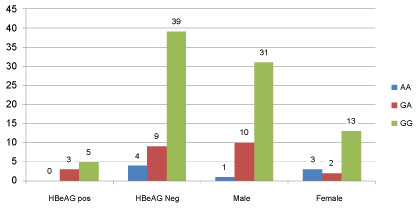 Figure 2: Frequency distribution genotype of rs10202545 in two groups HBe Ag positive and negative and different gender.
View Figure 2
Figure 2: Frequency distribution genotype of rs10202545 in two groups HBe Ag positive and negative and different gender.
View Figure 2
Table 2: Frequency of SNP alleles in Male and Female at different groups. View Table 2
Table 3: Frequency of SNP alleles in different groups. View Table 3
Table 4: Frequency of SNP alleles in different HBV Load levels. View Table 4
Hepatitis B virus (HBV) is one of the main causes of chronic liver infection. According to the WHO estimates, around 350 to 400 million people worldwide are infected with the Hepatitis B virus and each year about 500,000 to 1,200,000 die due to hepatitis B complications [20,21]. In Iran, it has been estimated that 1.5 million people have been diagnosed with hepatitis B, of which 15 to 45 percent are susceptive to cirrhosis and liver cancer [22]. Chronic hepatitis B (CHB) infection refers to a past inflammation of the liver at least six months and the person infected with HBV, HBs Ag remains positive for up to six months after infection [23]. Chronic hepatitis B remains for many years, even for decades. In many people, the disease is quite mild and does not cause any particular liver damage. However, in some patients, inflammation continues and causes the destruction of liver cells, which eventually becomes cirrhosis and liver cancer [24]. The main cause of the progression of hepatitis is chronic hepatitis, a person's immune system. In the event of a complete and correct immune response during an acute infection, it clears the HBV virus and, treatment. However, in the case of a defective immune system, acute infection will become a chronic infection [25]. PD-1 (CD279) is a receptor of the CD28/CTLA-4 family. The inhibitory signals provided by the PD-1 linkage and its ligands, PD-L1 (B7-H1; CD274) and PD-L2 (B7-DC, CD273), are provided to maintain the required balance for T-cell activation, Tolerance, and immune-mediated tissue damage, and inhibition of its function can reverse the function of T cells and their effective re-activity [26-28]. There are several studies that points to the role of PD-1/PD-L in preventing autoimmune disorders. It has been shown that the modified PD-1 function and its ligands are associated with several autoimmune diseases in humans [29-31]. Studies have shown that the frequencies of PD-1 genes and alleles in individuals with clinical symptoms of chronic hepatitis B are different from healthy subjects, and rs10204525 polymorphism play a predisposing role in the disease and severity [32]. Regarding the role of PD-1 in the chronic infection of hepatitis B in several studies, their association with the progression of infection, and SNP rs10204525 in the expression of PD-1 affects chronic hepatitis B infection is still not completely determined [33]. So, in this study, measured the level of PD-1 mRNA expression in PBMCs (Peripheral Blood Mononuclear Cells) and its association with the clinical parameters of hepatitis B infection and genotypes of the PD-1 rs10204525 polymorphism, which include three genotypes AA, GA and GG. In this study showed that in patients with chronic infectious HBV expression of PD-1 mRNA significantly increased incompared with healthy group. Increased expression level of the PD-1 mRNA was associated with increased levels of HBV DNA load and positive HBe Ag inserum. On the other hand, the viral load and HBe Ag represent the replication of the virus; therefore, these findings indicate that PD1 gene expression can play a role in the development of chronic infections [34]. Also, in this study, the levels of liver functional enzymes were tested, results have shown that SGOT, SGPT and ALK enzymes, were significantly related to the PD-1 mRNA expression (P < 0.001). As each increases of these enzymes, the expression of PD-1 mRNA also increases. In another part of the study, the relationship between SNPrs10204525 alleles with the level of liver enzymes was determined in patients with chronic hepatitis B and it was showed that the levels of all enzymes were significantly associated with GG allele of SNPrs10204525. In patients with chronic HBV infection, which have severe viremia and T cell dysfunction, PD-1 have a role in regulating of T cells [34,35]. PD-1 expression has a direct relation in T cells CD4+ and CD8+ with HBV DNA levels and an inverse relationship with T cells activated. In addition, HBV DNA levels and HBeAg indicate the high expression of PD-1 in chronic HBV infection [28,36]. Our research showed that the level of PD-1 expression in PBM cells was related to HBV DNA levels and HBeAg, which confirmed these findings and important role of the PD-1 in the HBV virus replication pathway, by suppressing host immune responses [37,38]. In previous studies, Zhou, et al. [32]. Have shown a close correlation between increased expression of PD-1 and high levels of HBV replication [32]. In a study showed that the level of PD-1 mRNA expression in patients with chronic hepatitis B was higher than the control group, there is no association between levels of PD-1 mRNA expression in peripheral blood nucleus and SGPT, SGOT, ALK in patients with chronic HBV infection [34]. Frequency of rs10204525 alleles in different geographic region of world is variable, in a study by Zhang, et al. [11]; the AA allele was the most frequent in CHB patients in China, but in a study by Hou and colleagues in other region of China, Showed that the GG allele is equal in healthy subjects and people with hepatitis B, but the AA allele in the healthy subjects and GA was highest in people with hepatitis B infection, However in our study frequency of GG allele in CHB group was higher than the control group, and this genotype was associated with a significant increase in the level of PD-1 mRNA, the difference between frequency of genotypes of rs10202545 can be due to racial and ethnic differences of individuals [11,39]. In our research and previous studies, there was a close relationship between increased expression of PD-1 and increased levels of HBV viral transcription. In addition, active viral transcription of HBV leading to disease progression in patients with chronic infections of HBV and increased expression of PD-1 on HBV specific T cells CD8+, CD4+ and T regulatory cells CD25, Causing a defect in the function of T cell response and continued HBV infection. Therefore, the negative effect of GA genotype on PD1 rs10204525 on chronic HBV infection and progression of the disease may be expressed, at least, through its association with the increase of PD-1 expression [40]. Association between a specific PD1 polymorphism and an increase in PD-1 expression, as well as clinical and viral symptoms, suggests that this polymorphism may potentially be used as a genetic marker to monitor the prognosis of chronic HBV infection. In addition, it has been shown that T-cell antiviral responses to chronic hepatitis B can be restored and improved by PD-1 pathway obstruction [40]. Therefore, this polymorphism may be a potential target for the design of immunotherapy interventions and the screening and selection of patients with HBV with a specific genetic immunological profile and will be effective in future approaches to immunotherapy for hepatitis B virus disease [41]. Further studies are definitely necessary to confirm the direct functional association between this polymorphism and the PD-1 molecule. Moreover, given that the findings of this study were only obtained from people of Kerman province, reproductive studies are required in different ethnic groups.
In conclusion, this study showed that in patients with chronic HBV infection serum PD-1 mRNA levels significantly increased associated with liver enzymes (SGPT, SGOT) and HBV viral replication (HBV DNA Load), increase expression of PD-1mRNA can effect on process of chronic infection of hepatitis B by facilitating the transcription of the HBV virus by an anti-cancer viral immune response, and this can be at least partially related to SNPrs10204525 in PD1 3' UTR. These data may provide immune histochemistry data to monitor the prognosis of diseases and design an approach to immunotherapy for HBV-related diseases. At the end, it is suggested that more attention be paid to other PD1 polymorphisms and their association with cases such as liver cancer in patients with hepatitis.
We declare that we have no conflict of interest.
This study was performed as an MSc thesis of Peyman Ghorbani, and it was financially supported by the GI-Liver Research center, Kerman University of Medical Sciences, Kerman, Iran and Department of medical microbiology, Laboratory of medical virology in Kerman, Iran.
This study was performed as an MSc thesis of Peyman Ghorbani, and it was financially supported by the GI-Liver Research center, Kerman University of Medical Sciences, Kerman, Iran and Department of medical microbiology, Laboratory of medical virology in Kerman, Iran.