Stress echocardiography is an established tool for clinical assessment and decision making-process in patients with Coronary Artery Disease (CAD). Current indications include diagnosis and assessment of patients with suspected CAD and intermediate pre-test probability of the disease, assessment of patients with already known CAD, and diagnosis and assessment of patients with non diagnostic ECGs or with inconclusive exercise ECG tests [1]. The most commonly reported figures for sensitivity and specificity are around 80% and 90%, respectively [1], although it is clear that the diagnostic value of stress echocardiography is widely exceeded by its prognostic value. Over the years, there have been continuous developments in methods and technology, as well as expansion of the technique to non-CAD patients. Our purpose was to check over current literature in the field, mainly referred to the last two years. Therefore, we have made a revision of articles published in the field of stress echocardiography for the diagnosis and management of patients with myocardial ischemia by using Pubmed information.
Some articles have revised the kind of patients studied over the years by stress echocardiography. All of them have observed that the percentage of patients with ischemia and with abnormal results have decreased over these last decades [2,3]. Also, the profile of the patients is currently more favorable than previously, with more females, patients with normal Left Ventricular (LV) function, and presentations with non coronary chest pain. These current characteristics have been also observed when ischemia detection was studied by other techniques such as myocardial perfusion imaging [4]. In a study of our centre this more benign baseline profile was associated to the same percentage of revascularizations, suggesting a lower threshold for deriving patients to these procedures, and also with lower mortality during follow-up [2].
European guidelines on stable Coronary Artery Disease (CAD) have been recently published. Stress echocardiography is recommended in these guidelines for patients with an intermediate pre-test probability of CAD (> 15%); for patients with lower probabilities no stress testing is recommended [1]. This is in contrast with American guidelines, which still consider stress echocardiography for patients with low pretest probability of CAD [5]. In a recent study of our institution we have found that the performance of Exercise Echocardiography (ExE) in patients with low pre-test probability of CAD and normal LV function barely increased outcome prediction [6]. In addition, the annualized major cardiac event rate was as low as 0.21% in this population, and the number of studies needed to detect a case with ischemia in at least 3 segments was as high as 26. Similar results have been found in populations from other countries with low pretest probabilities of CAD [7]. The low event rates and the high number of test needed to detect an abnormal case limit the reach of imaging in these patients.
On the contrary, in patients with intermediate pre-test probability of CAD (34 ± 23%) and stable angina, a new article [8] confirms the usefulness of ExE instead of exercise ECG testing for saving costs, although the event rates were similar in patients assigned to ExE or exercise ECG.
In patients with normal ExE (no resting or exercise-induced wall motion abnormalities), there could be clinical symptoms and/or ECG changes during the test that arise concerns. In a recent study including 7,127 patients with a normal ExE, we have observed that 12.5% of the patients had either clinical symptoms or ECG changes during exercise, whereas 102 (1.4%) had both abnormalities [9]. Although the annualized major cardiac event rate was similar irrespective of ECG/clinical symptoms (1.1-1.4%) the number of patients that ultimately were referred to angiography during follow-up was higher among those with clinical plus ECG positivity (28%), also paralleling the number of revascularizations. Annualized revascularizations were 9.2% in the group with clinical plus ECG positivity, 2.9% in the group with clinical or ECG positivity and just 1.4% in the group in whom both parameters were normal. These low percentage of major cardiac events and of positive angiographies might suggest a role for computed coronary tomography in selected cases with normal imaging when clinical or ECG exercise testing positivity arises doubts.
In another Spanish study dealing with 1640 patients with an ExE without ischemia, the independent predictors of events were resting LV ejection fraction, creatinine levels, SCORE risk or diabetes, chest pain during the test, and known CAD [10]. Thus, this study emphasizes that even among patients with a normal ExE, there are subgroups at higher risk. However, annualized event rates were very low (< 1%) for those with a negative ExE and a SCORE risk < 10.
Patients with stable symptoms found to have ischemia in at least 3 segments by stress echocardiography (approximately 10% of the myocardium) are commonly sent to coronary angiography with a view on revascularization, although this is based on scarce evidence, mainly coming from the COURAGE Serial Nuclear Sub study [11]. Results on outcome with this approach as compared to medical treatment alone are expected for the end of 2019, when the results of the ongoing ISCHEMIA study will be available [12].
Current guidelines recommend a non-invasive stress test preferably with imaging for patients evaluated for acute chest pain that have no recurrence of chest pain, normal ECG findings and normal levels of cardiac troponin [13]. However, although chest pain units developed under the philosophy of limiting the number of missing patients with CAD, there have been lately a lot of criticisms regarding the widespread use of imaging for them [14]. An analysis over 200,000 patients admitted for acute chest pain in different American hospitals found that the percent of admissions, angiographies and revascularizations were much higher in centers that performed the highest number of studies with imaging. However, it did not translate into a lower number of events during follow-up. The rate of subsequent myocardial infarction was similar in hospitals classified by the percent of imaging tests performed [15]. Again, doubts surge about the appropriateness of performing imaging in patients with low pre-test probability of CAD, presenting with acute chest pain. We have recently demonstrated that even in this scenario, a low pre-test probability of CAD as assessed by the CAD consortium rule [16] limits the value of imaging. In a series of 737 patients with low pretest probability of CAD, the probability of ECG or echocardiographic evidence of ischemia during exercise testing was as low as 2.6%, and the rate of events during a 6-month follow-up was 0.8%, consisting on just coronary revascularizations in all these patients [17]. Other more sophisticated scores have been built to limit the number of tests performed in patients with low risk. In one of them, we have found that the addition of some markers of risk such as the presence of diabetes, hypercholesterolemia, previous revascularizations, and abnormal ECG to the variables already assessed by the CAD consortium rule (age, sex, and type of chest pain), had value to avoid unnecessary exercise testing [18]. The end-points of this study were major cardiac events, positive exercise ECG, positive exercise echocardiography, or abnormal angiography. In the derivation set of 1473 patients employed to test the model we found that a determined cut-off value (0) avoided 1 of 4 tests (25%), at the expenses of missing 4 patients (1.8%) due to a positive ECG/echo during exercise.
There is wide information regarding the incremental diagnostic and prognostic value of stress echocardiography over clinical characteristics, resting echocardiography and exercise testing in different populations and scenarios, particularly for patients with already demonstrated CAD, intermediate pre-test probability of CAD, and/or resting ECG abnormalities [19-25]. In patients with right bundle branch block, the number of interpretable leads is sometimes limited. In a recent study we have found the value of peak treadmill exercise parameters to define outcome in these patients [26], particularly the presence of contractile reserve. Annualized event rates were 3.3% in patients with increase in LV ejection fraction > 5%, 4.7% in those with limited increase in LV ejection fraction (1-5%) and 8.2% in those with no increase.
Cortigiani, et al. recently found that ischemia detected by either wall motion analysis or coronary flow reserve during pharmacological stress predicted the occurrence of events in high risk asymptomatic subjects with diabetes mellitus [27]. Of note, ischemia was found in up to 25% of these asymptomatic subjects. These findings contrast with previous literature showing that screening, this time with anatomical imaging, is not worthy in asymptomatic diabetic patients [28]. There have been a number of studies performing assessment of coronary flow reserve during stress, not only with pharmacological stress, but also with bicycle exercise. In one of them, ischemia by wall motion analysis and ischemia by coronary flow reserve were complementary to define outcome [29].
As the age of the population is increasing worldwide, it is interesting to have data on stress echocardiography in the elderly. Gurunathan, et al. studied 374 octogenarians [30] and confirmed that ischemia was an independent predictor of events, associated with a 50% increase in all-cause mortality.
The prognostic value of ischemia exceeds anatomical information provided by angiography or coronary computed tomography [31]. In a study by Prada-Delgado, et al. [32] on patients with hypertension and absence of CAD on angiography, those who had ischemia during ExE had more than 3 times overall mortality, more than 5 times cardiac death, and more than 8 times cardiac failure during follow-up than those that had both normal angiography and normal exercise echocardiogram. The ongoing CIAO registry will provide more insights into the outcome of patients with ischemia during stress imaging in the absence of coronary obstructions, as assessed by a coronary computed tomography [12].
For long time there have been discussions on the detrimental or beneficial consequences of an elevated blood pressure with exercise. In a wide research of our institution including more than 10,000 patients we have found that an excessive blood pressure increase with exercise (i.e., an increase in systolic blood pressure with exercise ≥ 80 mmHg) was associated with better outcomes in patients with known or suspected CAD referred for ExE, after adjustment for other potential confounders [33]. Patients with an excessive blood pressure increase were less likely to have abnormal ExE results, and had a significantly lower risk of mortality and major cardiac events [33] without evidence of an increase in their risk of stroke [34].
Speckle tracking echocardiography seems to have found its place in the armamentarium of tools for assessing rest LV function [35], but not yet in the assessment of regional or global LV changes during stress echocardiography. In a recent study it was found that, first, longitudinal strain assessment was not better than visual assessment performed by experts, and second, there is also a need for expertise when it comes to the interpretation of speckle tracking images during stress [36]. Indeed, strain assessment achieved better results when performed and interpreted by experts instead of by beginners. Similar results were found in another study [37]. On the contrary, a study by Clemmensen, et al. in cardiac transplant patients observed that longitudinal strain changes during supine bicycle echocardiography were better than wall motion changes to detect cardiac allograft vasculopathy [38].
Although not likely ready for routine clinical use yet, strain assessment has provided interesting data into the pathophysiology of myocardial ischemia. For instance, tardokynesis has been completely characterized by strain in ischemic conditions [39]. In other studies from of our center [40,41] we have observed that torsion is diminished in patients with ischemia during exercise testing. Interestingly, this impairment of torsion is caused by impairment of basal rotation, whereas apical rotation may be even increased during ischemia. In one study, normal or increased apical rotation during exercise-induced ischemia, was accompanied by decreased circumferential strain at the same apical level (Figure 1), suggesting therefore a compensatory mechanism [41].
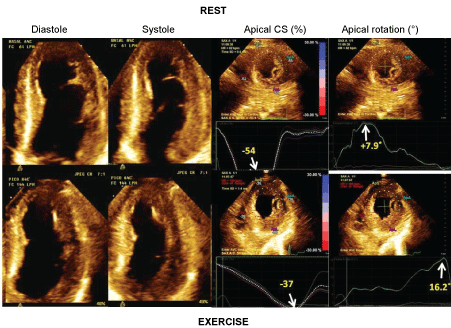 Figure 1: Example of a patient with severe ischemia. On the left, apical 4-chamber views in diastole and systole at rest and at exercise. On the right apical circumferential strains (%) and apical rotations (°) at rest (on the top) and at exercise (on the bottom). Note the increase in apical rotation along with the decrease in circumferential strain from rest to exercise.View Figure 1
Figure 1: Example of a patient with severe ischemia. On the left, apical 4-chamber views in diastole and systole at rest and at exercise. On the right apical circumferential strains (%) and apical rotations (°) at rest (on the top) and at exercise (on the bottom). Note the increase in apical rotation along with the decrease in circumferential strain from rest to exercise.View Figure 1
ExE may be useful for studying patients with dyspnea in the absence of abnormal findings in a resting echocardiogram. Increase in the ratio of early LV transmitral flow (E) to early tissue Doppler mitral annulus motion (e´) with exercise (with cut-off values of 13-15) may unveil exercise-induced LV diastolic dysfunction with high LV filling pressures as the cause of symptoms [42]. However, in up to 20-30% of patients evaluated for dyspnea, CAD may be the cause of the symptoms or a concomitant abnormality, and therefore regional and global LV function during exercise should also be tested [43,44]. A comprehensive approach for patients with dyspnea should include systolic and diastolic LV function and mitral regurgitation measurements, both at rest and at exercise (Figure 2).
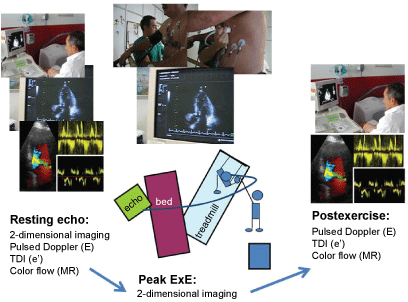 Figure 2: Treadmill exercise echocardiogram for the assessment of patients with dyspnea. At rest, traces for E/e´ assessment, mitral regurgitation signals and LV systolic function (regional and global) recordings are acquired. At peak exercise on the treadmill LV systolic function is assessed by a continuous imaging recording system. At post-exercise, E/e´ and mitral regurgitation are again assessed.View Figure 2
Figure 2: Treadmill exercise echocardiogram for the assessment of patients with dyspnea. At rest, traces for E/e´ assessment, mitral regurgitation signals and LV systolic function (regional and global) recordings are acquired. At peak exercise on the treadmill LV systolic function is assessed by a continuous imaging recording system. At post-exercise, E/e´ and mitral regurgitation are again assessed.View Figure 2
Patients with hypertrophic cardiomyopathy may develop different abnormalities during exercise such as an increase in LV filling pressures, LV outflow tract obstruction, mitral regurgitation due or not to systolic anterior motion of the mitral valve, and regional or global LV systolic dysfunction. Almost none of these abnormalities are commonly related to CAD in these patients. However we have observed that the presence of exercise-induced Wall Motion Abnormalities (WMAs) is an independent marker of outcome [45,46]. In one study, patients that had both exercise-induced WMAs and delayed hyperenhacement signal on a magnetic resonance study had the worse prognosis [46]. Also, in a recent multicentric study, it was observed that the best markers of poor outcome were those based on ischemia, as assessed by either WMAs assessment or coronary flow reserve, instead of haemodynamic markers such as exercise-induced obstruction or change in blood pressure [47].
Safety of stress echocardiography has been widely tested in single and multicenter studies. The frequency of serious complications is higher with pharmacological than with exercise stress echocardiography though [48]. In almost 20,000 ExE studies performed in our center we have had 19 (0.1%) serious complications [49] without any death, which is similar to previous figures reported in literature.
The choice of articles for this revision has mainly depended on novelty according to our view, and on whether they were or not appropriate for following our argumentative line. Of course there has been likely a bias to include articles published by our group, but we feel that it offers us the opportunity of putting them in context.
Over the years there have been confirmations on the role of stress echocardiography for risk stratification of patients with known or suspected CAD and different clinical characteristics and presentations (chronic, acute) but mainly for those with an intermediate pretest probability of the disease. Strategies to avoid the widespread use of this technology in other subsets of patients should be encouraged. Ischemia has been also recognized as a marker of worse outcome in patients without CAD.
There is no conflict of interest to disclose.