Objective: To compare the Free Style Libre (FSL) 2 14-day Flash Glucose Monitoring (FGM) and Point-Of-Care Capillary (POC) Blood Glucose (BG) testing among critically ill patients who receive insulin infusion.
Methods: We conducted an open-label, prospective study and enrolled stepdown or critical care unit patients on intravenous insulin. Paired POC and GCM values were obtained to calculate the Mean Absolute Relative Difference (MARD). Accuracy was further evaluated with error grid analysis between matched glucose pairs.
Results: Using POC BG as the reference, the overall MARD was 8.65%. For POC BG of 70-180, MARD was 10.02%, whereas for POC BG of > 180 mg/dL, MARD was 6.74%. Error grid analysis showed 99.9% of glucose pairs within zones A and B.
Conclusion: FSL2 exhibits satisfactory accuracy in critically ill patients receiving insulin infusion, suggesting the future use of FGM in guiding insulin dosing for this subgroup of patients.
Self-monitoring of blood glucose, Continuous glucose monitoring, Flash glucose monitoring, Diabetes mellitus, Ketoacidosis
FSL: Free Style Libre; FGM: Flash Glucose Monitoring; POC: Point-of-Care capillary glucose testing; BG: Blood Glucose; DKA: Diabetic Ketoacidosis; HHS: Hyperglycemic Hyperosmolar Syndrome patients; MARD: Mean Absolute Relative Difference
In patients who present with acute metabolic emergencies such as Diabetic Ketoacidosis (DKA), Hyperglycaemic Hyperosmolar State (HHS), frequent finger sticks are required to obtain POC BG values for monitoring and titration of the insulin infusion. Continuous Glucose Monitoring (CGM) devices, which have been used in both type 1 and type 2 diabetes mellitus, have been shown to decrease hospitalization for diabetic ketoacidosis (DKA) [1] and are a safe substitute for Point of Care blood glucose (POC BG) monitoring [2]. There are currently 2 types of CGM systems: real-time CGM (rtCGM) and flash GM (FGM). The FreeStyle Libre 2 (FSL2) is the newest flash CGM system commercially available. FDA has not approved the use of FSL in critically ill patients [3]. Recent study showed that FSL2 is safe and accurate in inpatient non-critical care setting [4]. However, it remains unclear whether FGM can safely be used for insulin adjustment in the critical care setting. We report the first study evaluating the safety and accuracy of the 14-day FSL2 in the critical care setting, and provide data for future study to evaluate the practicality of FGM in stepdown unit and Intensive Care Unit (ICU).
We compared the matched-pair FreeStyle Libre CGM system® (Abbott U.S.) and capillary Point-of-Care (POC) glucose data from patient placed on nursing driven continuous insulin infusion protocol in either the stepdown unit or Intensive Care Unit (ICU). Patients were eligible if they had blood glucose > 320 mg/dL on admission in the emergency department, met requirement for DKA, HHS, severe hyperglycemia, or required insulin infusion for underlying condition (e.g. hypertriglyceridemia-induced pancreatitis). Patients remained eligible if they were receiving mechanical ventilation, vasopressors, or renal replacement therapy (RRT). Patients were excluded if they were receiving high-dose ascorbic acid or salicylic acid [5].
Descriptive data are presented as mean ± SD for continuous variables (age, HbA1c) and count (%) for categorical variables. Parameters for estimating accuracy included Mean Absolute Relative Difference (MARD), and proportion of CGM values within 15, 20, and 30% or 15, 20, and 30 mg/dL of POC reference values for blood glucose > 100 mg/dL or ≤ 100 mg/dL, respectively (%15/15, %20/20, %30/30). Mean average relative difference (MARD) is calculated between measurements of POC glucose and FCGM values. Clinical reliability was assessed with Clarke error grid (CEG) analyses [6]. In CEG, region A describes all values that are within 20% of the reference glucometer; region B describes points that are outside of the 20% but would not lead to inappropriate treatment; region C describes points whose errors would lead to unnecessary treatment; region D describes points in which reliance on the test glucometer (but not the reference glucometer) would lead to potentially dangerous failure to detect hypoglycemia or hyperglycemia; region E describes points that would confuse treatment of hypoglycemia for hyperglycemia and hyperglycemia for hypoglycemia. All analyses were performed in STATA 14.
A total of 9 patients were included (5 out of 9 with type 2 diabetes) with a mean age of 41.8 ± 14.8 (standard deviation) years, an average body mass index (BMI) of 30.7 ± 5.9 kg/m2, and a mean hemoglobin A1c 12.2 ± 2.5%. Five out of 9 were female; 1 had hypertriglyceridemia-induced pancreatitis, 1 had uncontrolled gestational diabetes, 1 was in circulatory shock and had severe hyperglycemia, 2 had HHS and 4 had DKA.
A total of 91 paired glucose readings were obtained. Using POC BG as the reference, the overall MARD (n = 91) was 8.65%. For POC BGs of 70-180 mg/dL, the MARD was 10.02%; for POC BGs of of > 180 mg/dL, the MARD was is 6.74%. Among the POC BG readings > 100 mg/dL, the percentages of glucose readings within ± 15%/15 mg/dL, ± 20%/20 mg/dL, and ± 30%/30 mg/dL were 67.47%, 77.11%, and 89.16%, respectively. The Pearson correlation was 0.96. CEG (Figure 1) analysis showed 99.9% of glucose pairs within regions A and B, with no values in regions C, D, and E.
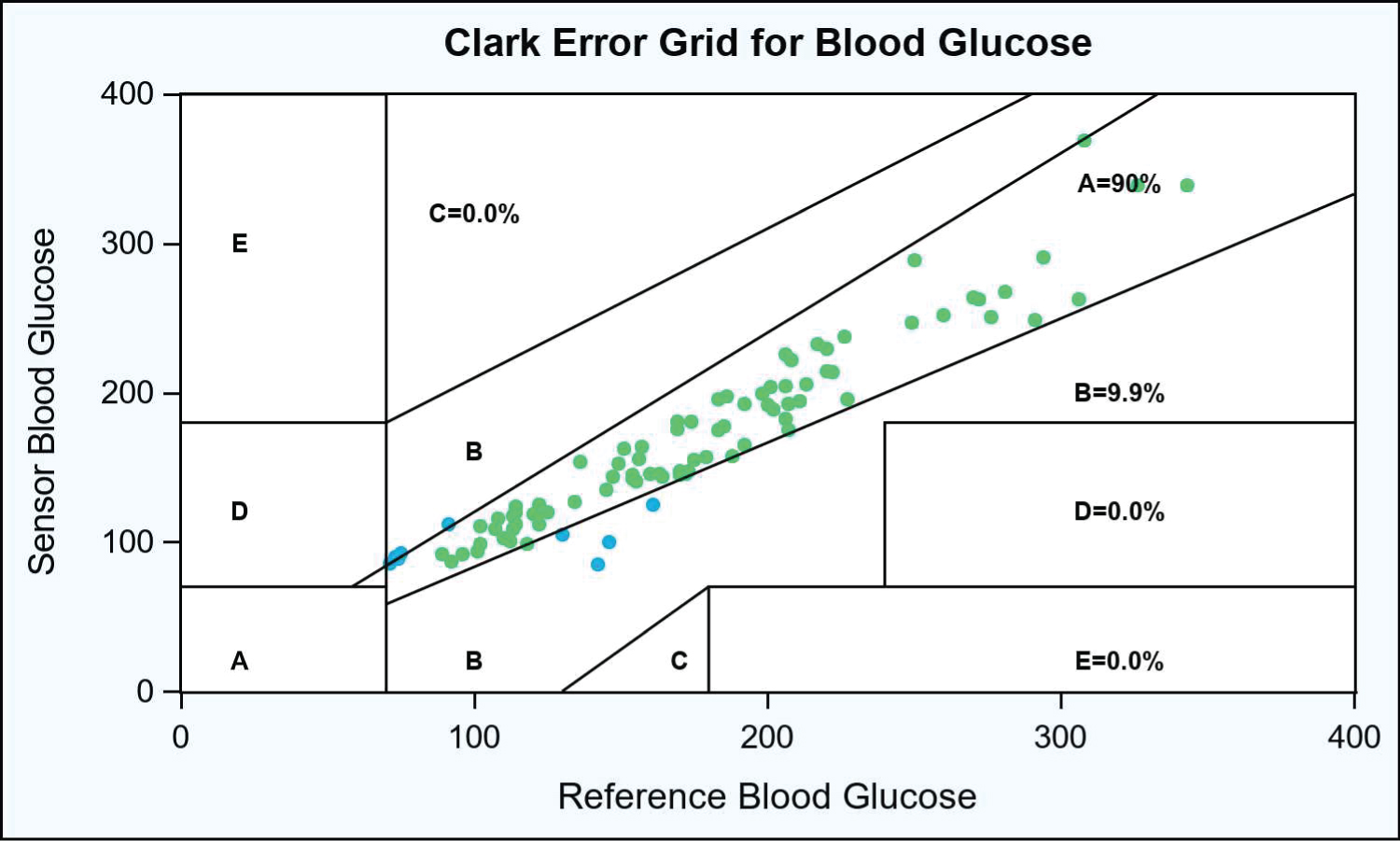 Figure 1: Clark error grid analysis for blood glucose.
View Figure 1
Figure 1: Clark error grid analysis for blood glucose.
View Figure 1
In this Figure 1, each dot (n = 91) denotes a pair of blood glucose readings taken from patients (N = 9) in the stepdown unit or intensive care unit. Dots in green denote paired readings that fall in Region A (values within 20% of the reference glucometer), whereas dots in blue denote paired readings that fall in Region B (values that are outside of the 20% but that would not lead to inappropriate treatment). Please refer to the methods section for a full description of the CEG regions.
Inpatient use of FGM could improve patient care and nursing workflow, especially in critical care units, but its safety and accuracy are not established. To our knowledge, this is the first study to demonstrate the practical application and accuracy of FSL2 in critically ill patients on insulin infusion.
First, we established a high accuracy of FSL2, evidenced by a MARD of 8.65% and CEG of 99.9% in zone A and B. In a non-critical inpatient setting, acceptable MARD ranged from 14% to 15.6% [7,8] whereas in critical care setting, no acceptable MARD has been defined yet. Studies suggested that MARD of < 14% is acceptable, a MARD > 18% represents poor accuracy [9] and that lowering of MARD ≤ 10% from reference values did not improve glycemic outcomes [10].
Second, we demonstrated that FSL use is feasible in the setting of step-down unit or ICU. FSL use in an outpatient setting reduced acute events of DM including HHS and DKA [11]. FSL use in inpatient non-critical setting had a MARD of 17.8% with capillary BG of 180-250 mg/dL and 19% with capillary BG of > 250 mg/dL [4]. Our MARD was 6.74% with BG > 180 mg/dL. This discrepancy could potentially due to the longer time allowance for paired values (15 mins by Wright, et al. [4]) than our studies. We encouraged nurses to obtain the paired BG values simultaneously to minimize disruption of the hectic workflow of critical care unit. Our patients were critically ill, and likely had systemic inflammation that results in increase in capillary permeability, which facilitate diffusion of the intravascular glucose to the interstitial space. Therefore, the lag time between interstitial and venous BG is shortened, and improved the accuracy of FSL. Besides, the lag time is more problematic in the post prandial state than the fasting state. Most of our patients were fasting due to underlying conditions. Hence, there were less prandial fluctuations and better correlations between CGM and POC BG. In the studies conducted by Nair, et al. [12] in 10 patients who were fasting for surgery and received either intravenous (IV) or subcutaneous (SQ) insulin therapy, the MARD between CGM and POC was 9.4% which is compared to our findings.
A recent study evaluated a group of DKA patients using FSL1, and reported no adverse events related to sensor use [10]. There is no Randomized Control Trial (RCT) on the safety and efficacy of CGM in guiding insulin adjustment in critically ill patient who receives insulin infusion. There was a RCT that used Dexcom G6 CGM to guide basal bolus insulin therapy, and showed no difference in total daily insulin dose between POC and CGM groups [13]. The main barrier to the use of CGM devices in critically ill patients who are often in shock, edematous and acidotic, is the accuracy and safety of CGM [14]. Recent studies challenged this predisposition, albeit in small sample and unblinded setting. Villard, et al. demonstrated that among dialysis patients who commonly are fluid overloaded, the MARD of CGM is comparable to SMBG, with 98.7% of the value in error grid A, indicating high accuracy of CGM in dialysis [15]. Perez-Guzman, et al. reported a MARD of 12.9% in ICU patients, some of whom were in shock [16]. Agarwal, et al. reported a MARD of 12.58% in patients on renal replacement therapy, vasopressors, mechanical ventilation support [17].
We enrolled critically ill patients with a variety of medical complications (e.g shock, respiratory failure on ventilator support, DKA, HHS, etc) and established the accuracy of FSL2 in a real-world setting.
Our study sample is small, as our study aims provide pilot data for a future RCT. As this was an open-labelled study, it is subject to inherent bias. Future studies should evaluate if reliance of FGM in place of fingerstick-based POC BG leads to improved healthcare delivery and patient satisfaction.
In conclusion, there appears to be an acceptable statistical agreement between FSL2 and POC BG readings. Our results provide a proof of concept that FGM can be used safely and effectively during critical illness, while offering reliable accuracy and decreasing POC glucose testing frequency.
None.
None.
None.