Polycystic Ovary Syndrome (PCOS) is a common endocrine disorder, present in 5-13% women of reproductive age. The endocrine manifestations of PCOS include excess androgen production of ovarian and/or adrenal origin and arrested follicular development leading to chronic oligo- or anovulation. Long term health risks of women with PCOS include cardiovascular disease, type 2 diabetes mellitus and endometrial cancer. PCOS is diagnosed by chronic anovulation, polycystic ovaries on ultrasound and biochemical/clinical manifestations of hyperandrogenism. Phenotype expression is heterogeneous and varies throughout the woman's life cycle, making early confirmation difficult. South Asians with anovulatory PCOS manifest severe symptoms at a younger age, with greater insulin resistance and a higher prevalence of the metabolic syndrome than white Europeans, thereby reflecting their ethnic propensity to type 2 diabetes mellitus. PCOS appears to be a multigenic trait, although contributing genes remain undefined yet. Several studies have been carried out to identify the candidate genes and polymorphisms affecting the multiple biological pathways of PCOS. The main objective of this article is to review the role of genes regulating the Hypothalamus-Pituitary-Gonadal (HPG) axis - mainly KISS1, GPR54 receptor gene, GnRH (Gonotropin Releasing Hormone), GnRHR (Gonotropin Releasing Hormone Receptor), FSH (Follicle Stimulating Hormone), FSHR (Follicle Stimulating Hormone Receptor), LHβ (Luteinizing Hormone beta subunit) and LHCGR (Luteinizing Hormone/Choriogonadotropin receptor) genes; with special focus on its association with PCOS.
Hypothalamic pituitary gonadal axis, Polycystic ovary syndrome, South Asia
Polycystic Ovary Syndrome (PCOS) is a common endocrine disorder, affecting women of reproductive age with prevalence varying between 5-13%. PCOS typically presents during adolescence with a wide spectrum of phenotypes that are characterized by features of anovulation (amenorrhoea, irregular cycles) combined with symptoms of androgen excess (hirsutism, acne, alopecia) and polycystic ovaries on ultrasound [1]. The characteristic biochemical abnormalities are elevation of serum androgen concentrations (particularly testosterone and androstenedione) and Luteinizing Hormone (LH) concentrations, but with normal or low levels of Follicle-Stimulating Hormone (FSH) [2]. Diagnosis of PCOS is based on the 'Rotterdam criteria', which require the presence of two of the three following features: polycystic ovaries, anovulation and androgen excess (clinical and/or biochemical) [3].
Current studies suggest that excess androgen production may induce the polycystic ovarian morphology, leading to the endocrine disruption of this disorder. In addition, presentation of PCOS in adolescence suggests that there is an underlying predisposition to the typical ovarian abnormalities that has origins before the onset of puberty. The basis of these two hypotheses was obtained from studies done by Abbott and colleagues on female Rhesus monkeys. In this study, the animals were exposed to high concentrations of testosterone in uterus and when they developed as adults showed typical features of PCOS such as hypersecretion of LH, ovarian hyperandrogenism, anovulation in relation to increased body weight and insulin resistance [4,5]. This shows that PCOS is induced by excess androgens and may arise by 'programming' of the hypothalamic-pituitary-ovarian axis by androgens in prenatal life [6].
PCOS is associated with metabolic abnormalities, central to which are insulin resistance and hyperinsulinemia, which carry an increased risk of developing type 2 diabetes in later life [2]. In addition, PCOS is one of the leading causes of female infertility. Lifestyle changes, such as losing weight, can trigger body changes that facilitate conception in women with PCOS [7].
Based on current research on PCOS there is strong evidence that genetic factors play a major role in its etiology. Despite several genetic studies dissecting the variants of genes from multiple biological pathways in its pathophysiology, the mode of inheritance of PCOS remains unclear [8]. Current findings favor PCOS as a complex endocrine disorder that results from the interaction of susceptible and protective genomic variants in several genes under the influence of environmental factors [9-11]. Candidate genes involved in steroid hormone metabolism, gonadotropin and gonadal hormones action, obesity and energy regulation, insulin secretion and action have been studied and implicated in the pathogenesis of PCOS. There may be several interlinking factors that affects expression of PCOS. A single cause for PCOS is unlikely [12]. Moreover, a study on phenotype genotype correlation in PCOS revealed that a PCOS genetic gradient resulted from genetic drifts due to a serial founder effect that occurred during ancient human migrations. The overall prevalence of the disease supports intra-locus sexual conflict as alternative to the natural selection of phenotypic traits in females [13].
We believe that focusing on the Hypothalamus-Pituitary-Gonadal (HPG) axis related genetic polymorphisms may shed fresh light on providing a more complete picture on the genetic basis of PCOS. Heritability can be studied by 4 methods - twin studies, family association studies, candidate gene studies and Genome-Wide Association Studies (GWAS). In this review we have analyzed the heritability based on the candidate gene studies related to the HPG axis and PCOS.
In our review, the role of genes regulating the HPG axis - mainly KISS1, GPR54 receptor gene, GnRH (Gonotropin Releasing Hormone), GnRHR (Gonotropin Releasing Hormone Receptor), FSHβ (Follicle Stimulating Hormone beta subunit), FSHR (Follicle Stimulating Hormone Receptor), LHβ (Luteinizing Hormone beta subunit) and LHCGR (Luteinizing Hormone/Choriogonadotropin receptor) genes; with special focus on their association with PCOS were selected. Several studies have been carried out to identify the association of polymorphisms of these genes with polycystic ovary syndrome. However, repeatability of results has remained low.
Female reproductive function depends on the proper development and regulation of the HPG axis. Kisspeptins are peptide products of KISS1 gene that participate in the control of the HPG axis. Kisspeptin act via G protein-coupled receptor known as GPR54 [14,15]. The GPR54 - KISS1 pathway has an essential role in the initiation and maintenance of mammalian fertility [16].
The KISS1 gene is localized on chromosome 1q32 and consists of three exons, of which only part of the second and third exons are finally translated into a precursor 145 amino acid peptide, which is cleaved into three forms of kisspeptins containing 54, 14, or 13 amino acids. The three peptides exhibit the same affinity for their single receptor (GPR54) since they share a common C-terminal decapeptide. GPR54 gene maps to chromosome 19p13.3 and includes five exons, encoding a 398 amino acid protein with seven hydrophobic trans-membrane domains [17].
The KISS1 gene was originally identified in 1996 as a suppressor of metastasis in human malignant melanoma [18]. The role of Kisspeptin in reproduction was identified in 2003, which revealed the current understanding of the neuroendocrine regulation of human reproduction and the role of kisspeptin in puberty [19]. Kisspeptin signals directly to the GnRH neurons through the action on the kisspeptin receptor (GPR54) to release GnRH into the portal circulation, which in turn stimulates the secretion of LH and FSH from the gonadotrophs of the anterior pituitary [20]. GnRH secretion is deregulated in PCOS. Therefore, it can be postulated that altered patterns of kisspeptin inputs to GnRH neurons leads to dysregulated gonadotropin secretion in PCOS.
The most important function of the KISS1/GPR54 system in the process of puberty makes it necessary to investigate the mutations and polymorphisms in the KISS1 and GPR54 genes and their association with PCOS. However, polymorphisms in KISS1 and GPR54 genes in relation to PCOS are not well studied. Therefore, further research should focus on identifying the polymorphisms in KISS1 and GPR54 genes and their expression levels in relation to PCOS and determine the association of these polymorphisms with clinical, biochemical and endocrine features in order to obtain a complete view of the scenario predisposing to PCOS. This will help expand our understanding of the basis of KISS1 and GPR54 genes in PCOS.
The KISS1 gene encodes kisspeptin that signals directly to the GnRH neurons through the action on the kisspeptin receptor (GPR54) to release GnRH into the portal circulation, which in turn stimulates the secretion of LH and FSH from the gonadotrophes of the anterior pituitary by binding to its receptor GnRHR-1. LH and FSH act on gonads (by binding to their receptors LHCGR and FSHR respectively) and stimulate the release of estrogen, testosterone and progesterone (Figure 1).
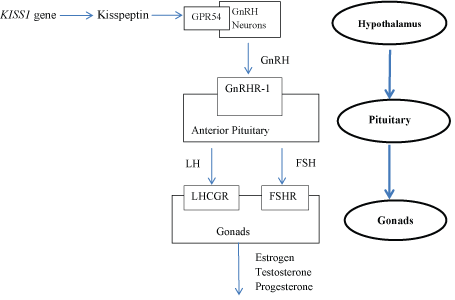 Figure 1: Diagrammatic representation of the HPG axis pathway in humans.
View Figure 1
Figure 1: Diagrammatic representation of the HPG axis pathway in humans.
View Figure 1
The GnRH and its receptor (GnRHR) genes are important regulators of the HPG axis, and abnormalities in their function lead to impaired pubertal development and sexual maturation [21].
GnRH is a neurohormone consisting of 10 amino acids (decapeptide) that is produced in the arcuate nuclei of the hypothalamus [22]. GnRH stimulates the secretion of the two gonadotropins-LH and FSH by the anterior pituitary gland which stimulate spermatogenesis, folliculogenesis and ovulation. In addition, gonadal steroid hormones androgen, estrogen and progesterone participate in the negative feedback loop and inhibit GnRH and gonadotropin expression [23]. Four different GnRHs are reported to be expressed in various mammals. Of the four GnRHs, only GnRHI (mammalian GnRH) and GnRHII (chicken GnRH II) genes were identified in the human genome [24].
The GnRH1 gene is located on chromosome 8p21.2. It spans about 5 kb and contains 3 exons. It encodes the GnRH1 precursor, which contains 92 amino acids, and it is subsequently processed to GnRH1, an active decapeptide. GnRH is the principal hormone regulating the pituitary gonadotropins, there by affecting the ovarian physiology. Lack of negative feedback regulation on GnRH pulse frequencies, can lead to excess secretion of LH; which in turn increases androgen biosynthesis in ovarian theca cells and results in hyperandrogenism, a key etiological factor in the pathogenesis of anovulation and infertility in PCOS women [25].
The GnRHR gene is located on chromosome 4q13.2. Its genomic sequence covers about 19 kb and it includes 3 exons. GnRHR gene encodes the receptor for GnRH1 hormone. This receptor is a member of the seven-transmembrane, G-protein Coupled Receptor (GPCR) family. It is expressed on the surface of pituitary gonadotrope cells as well as lymphocytes, breast, ovary, and prostate. Following binding of GnRH1, the receptor associates with G-proteins that activate phosphatidylinositol-calcium second messenger system and activation of the receptor leads to the secretion of LH and FSH [23].
So far, no major defects within GnRH1 and GnRHR genes have been found in association with PCOS. However, a polymorphism in the first exon of GnRH1 has been described, constituting an amino acid variation at codon 16 (Trp16Ser). A study by Valkenburg, et al. [26] showed distribution of the Trp16Ser alleles of GnRH1 was similar in PCOS cases and controls and failed to identify any association with PCOS. Therefore, we can conclude that GnRH1 and GnRHR genes mutations are uncommon in subjects with PCOS.
Follicle Stimulating Hormone (FSH) is a glycoprotein secreted by the anterior pituitary. It is a heterodimer consisting of common α-subunit and a hormone-specific β-subunit that contributes to the receptor binding specificity [27]. FSH secretion is regulated by GnRH and in turn regulates gonadal functions in males and females by activating their cognate receptors. In women, it plays a crucial role in the follicle development, oocyte maturation, steroidgenesis regulation, proliferation of granulosa cells and induces synthesis of the androgen-converting enzyme aromatase [28].
The effect of FSH is mediated by binding to its receptor - FSHR (Follicle Stimulating Hormone Receptor), which is specifically situated on the granulosa cells of the ovary [29]. FSHR gene is located on chromosome 2p21 and comprises 10 exons and 9 introns.
The FSH and FSHR genes are necessary for female fertility. The importance of FSHR in the signaling transmission of FSH made FSHR gene one of the important candidate genes for PCOS [30]. More than 900 SNPs in the FSH and FSHR genes have been reported [31]. Mutations in FSHR gene can lead to arrest of follicle development at several phases of growth [32,33]. Genetic variants in the FSHR gene may have effects on the phenotype. These effects include variable development of secondary sex characteristics, primary amenorrhea, hypoplastic ovary, and high serum FSH levels [34,35]. The two most clinically relevant polymorphisms of FSHR gene are in exon 10. One polymorphism is located at codon 307 in the extracellular domain of the receptor, where alanine is replaced by threonine (A307T; rs6165). The other polymorphism is in the intracellular domain at codon 680, where asparagine is replaced by serine (N680S; rs6166) [34,36].
The association between FSHR gene polymorphisms and PCOS were examined by several studies but the results were contradictory. Gu, et al. reported Ser680Asn of FSHR gene polymorphism was associated with PCOS in Korean women, whereas the Ala307Thr was not [37]. Meanwhile Dolfin, et al. showed that the Ala307Thr of FSHR polymorphism was related to PCOS in Italian women [38]. In addition, Unsal, et al. found that the genotype frequencies of the Ala307Thr and Ser680Asn polymorphisms of FSHR were not different between cases and controls in Turkish adolescent girls [39]. Sudo, et al. reported a significant increase in the Ala307Thr frequency among Japanese women with PCOS when compared to normal subjects [40]. A significant association between the polymorphism Ala307Thr and PCOS was also reported by a recent study on Chinese women [41]. However, Mohiyiddeen, et al. did not find any association between the Ser680Asn polymorphism of FSHR gene and PCOS in a British population [42]. In addition, Valkenburg, et al. concluded that FSHR gene variants were strongly associated with the severity of PCOS clinical features, but not with disease risk [26]. Orio, et al. reported no significant relationship between various polymorphisms of FSHR gene and PCOS in Italian females [43]. Contradictory findings of the different studies may be due to the variation in sample size and ethnicity of the study population.
Importantly, most studies have focused on association of FSHR gene polymorphism with PCOS, whereas association of FSHβ gene polymorphism with PCOS is less explored. Tong, et al. concluded that FSHβ gene mutations were found to be uncommon in patients with PCOS. However, AccI polymorphism was found to be associated with the syndrome in some women, especially those with obesity and hyperandrogensim [44].
Luteinizing Hormone (LH) is a member of the glycoprotein hormone family that includes human Chorionic Gonadotropin (hCG), FSH, and Thyroid Stimulating Hormone (TSH). They are α/β heterodimers with a common α -subunit and a unique β -subunit. The β -subunit confers biologic specificity [45,46]. The two LH subunits (α and β) are encoded by separate genes. The α subunit gene is in chromosome 6 and the β subunit gene is in chromosome 19 [47]. LH stimulates follicular development, steroidogenesis, and the formation of corpus luteum [48], and ovulation results from a surge in LH levels [49]. LH acts by binding to its high affinity receptor known as Luteinizing Hormone/Choriogonadotropin receptor/Choriogonadotrophin receptor (LHCGR) [48]. LHCGR is a G protein-coupled hormone receptor and is expressed in numerous tissues including the gonads, uterus [50], fallopian tubes [51], placenta and fetus [52]. Both LH and hCG are endogenous ligands for LHCGR [53]. LHCGR is encoded by LHCGR gene located on chromosome 2p21 and composed of 11 exons occupying about 700 kbp [54].
The relationship between LH signaling pathway and PCOS has not been clearly understood. However, abnormal LH signaling is believed to play a significant role in augmenting ovarian androgen production in PCOS leading to anovulation [55]. LHβ and LHCGR gene mutations may change the structure or function of the LH and LHCGR, either activating or inactivating their bioactivity, which cause anovulation, amenorrhea, and polycystic ovaries in women [56]. Several studies have proven the genetic associations of LHβ and LHCGR polymorphisms with PCOS, although the results of different populations and loci of polymorphisms showed inconsistencies.
There are 2 common mutations of LHβ gene that were associated with PCOS; one in codon 8 and other in codon 15. Point mutation in codon 8 causes amino acid replacements from Trp to Arg; and in codon 15 from Ile to Thr [57]. Kurioka, et al. showed that these mutations were considerably associated with PCOS [58] and Tapanainen, et al. concluded that the presence of these mutations may help to diagnose the risk for PCOS particularly among obese women [59]. However, studies in a British population revealed that the incidence was not higher in women with PCOS, though it was increased in obese women with PCOS [60]. In contrast, Huhtaniemi, et al. reported that variant LH occurs with normal frequency in non-obese patients with PCOS but is underrepresented in obese patients with PCOS [61]. These results show that the clinical significance of the variant LH with respect to PCOS is contradictory. The same mutations were reported from Finland [62] and a similar LH form was described from Japan [57], which suggests that this variant LH represents a universal polymorphism [62]. Worldwide carrier frequency of this common genetic variant has been analyzed and reported as a prevalence of 7% in U.S. Hispanics, 18% in England and 42% in Lapps of northern Finland [60,62,63]. However, these studies also reported that the LH variant is less common in Asian countries.
The LHCGR gene has at least 300 known polymorphisms [45,64]. A study by Capalbo, et al. on S312N (G935A) polymorphism in exon 10 of the LHCGR gene revealed that this variant is strongly linked with PCOS in the Sardinian population [54]. The finding of this study was supported by two other studies by Ha, et al. [65] and Bassiouny, et al. [66]. They found that the G935A polymorphism of LHCGR gene is associated with PCOS in Hui Chinese and Egyptian populations. On the contrary, Almawi, et al. [67] and Valkenburg, et al. [26] reported that G935A polymorphism was not overtly associated with PCOS in Bahraini Arab and Caucasian populations. Furthermore, Thathapudi, et al. revealed that the GG genotype, rather than AA, conferred a significant risk for developing PCOS in South Indian women [68].
Eriksen, et al. found an association with rs13405728 variant in the LHCGR gene and PCOS in white Europeans of Danish origin and have suggested that the gene products of the LHCGR gene is linked with the diagnosis of PCOS, despite ethnicity [69-71]. Valkenburg, et al. found that LHCGR 18insLQ insertion allele frequency was significantly lower in white Europeans with PCOS than controls [26].
Table 1 summarizes the major findings of the candidate genes of the HPG axis and their association with PCOS.
Table 1: Comparison of genotype associations with PCOS. View Table 1
PCOS appears to be a multigenic trait, although the contributing genes broadly remain undefined. This review explored in-depth the possible associations between PCOS and genetic polymorphisms of the many genes linked to the HPG axis and found the reported associations to be conflicting. The discrepancy of findings is likely to be due to variations in study design, sampling technique and sample size, along with demographic and genetic differences among the study populations.
We propose the need to analyze polymorphisms in multiple candidate genes of PCOS to determine its exact genetic basis. Development of a genetic diagnostic tool that would help in screening multiple candidate genes simultaneously is suggested. Such a tool would also help elucidate which of these SNPs are present in the different phenotypic subgroups of affected women. Such an approach would help foster a better understanding of the genetic basis for the pathophysiology underlying PCOS in different subgroups and populations. This knowledge could then be leveraged to devise the most optimal screening and effective management strategy for an affected woman, depending on her phenotypic subgroup and ethnicity.
Our research is funded (financial and material support) by National Research Council of Sri Lanka (NRC grant no: 15-149).
None.
No conflict of interest.